Condensation polymers are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as byproducts such as water or methanol. Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization, when the polymer is formed by sequential addition of monomers to an active site in a chain reaction. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers.
A monomer is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.

A thermoplastic, or thermosoftening plastic, is a plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Thermal depolymerization (TDP) is the process of converting a polymer into a monomer or a mixture of monomers, by predominately thermal means. It may be catalysed or un-catalysed and is distinct from other forms of depolymerisation which may rely on the use of chemicals or biological action. This process is associated with an increase in entropy.

A thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure, or mixing with a catalyst. Heat is not necessarily applied externally, but is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their production, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.

Polyimide is a polymer of imide monomers belonging to the class of high performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.
Polyamide-imides are either thermosetting or thermoplastic, amorphous polymers that have exceptional mechanical, thermal and chemical resistant properties. Polyamide-imides are used extensively as wire coatings in making magnet wire. They are prepared from isocyanates and TMA in N-methyl-2-pyrrolidone (NMP). A prominent distributor of polyamide-imides is Solvay Specialty Polymers, which uses the trademark Torlon.

Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.

Isosorbide is a bicyclic chemical compound from the group of diols and the oxygen-containing heterocycles, containing two fused furan rings. The starting material for isosorbide is D-sorbitol, which is obtained by catalytic hydrogenation of D-glucose, which is in turn produced by hydrolysis of starch. Isosorbide is discussed as a plant-based platform chemical from which biodegradable derivatives of various functionality can be obtained.

CR-39, or allyl diglycol carbonate (ADC), is a plastic monomer commonly used in the manufacture of eyeglass lenses alongside the other material PMMA.

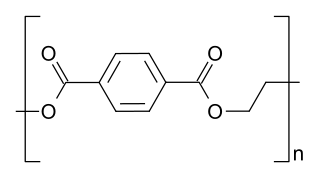
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
Synthetic resins are industrially produced resins, typically viscous substances that convert into rigid polymers by the process of curing. In order to undergo curing, resins typically contain reactive end groups, such as acrylates or epoxides. Some synthetic resins have properties similar to natural plant resins, but many do not.
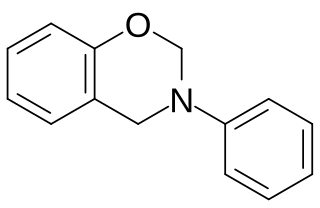
Polybenzoxazines, also called benzoxazine resins, are cured polymerization products derived from benzoxazine monomers.
Depolymerization is the process of converting a polymer into a monomer or a mixture of monomers. This process is driven by an increase in entropy.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the space shuttle.

In chemistry, hexahydro-1,3,5-triazine is a class of heterocyclic compounds with the formula (CH2NR)3. They are reduced derivatives of 1,3,5-triazine, which have the formula (CHN)3, a family of aromatic heterocycles. They are often called triazacyclohexanes or TACH's.
Vitrimers are a class of plastics, which are derived from thermosetting polymers (thermosets) and are very similar to them. Vitrimers consist of molecular, covalent networks, which can change their topology by thermally activated bond-exchange reactions. At high temperatures they can flow like viscoelastic liquids, at low temperatures the bond-exchange reactions are immeasurably slow (frozen) and the Vitrimers behave like classical thermosets at this point. Vitrimers are strong glass formers. Their behavior opens new possibilities in the application of thermosets like as a self-healing material or simple processibility in a wide temperature range.
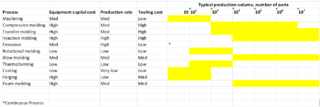
The economics of plastics processing is determined by the type of process. Plastics can be processed with the following methods: machining, compression molding, transfer molding, injection molding, extrusion, rotational molding, blow molding, thermoforming, casting, forging, and foam molding. Processing methods are selected based on equipment cost, production rate, tooling cost, and build volume. High equipment and tooling cost methods are typically used for large production volumes whereas low - medium equipment cost and tooling cost methods are used for low production volumes. Compression molding, transfer molding, injection molding, forging, and foam molding have high equipment and tooling cost. Lower cost processes are machining, extruding, rotational molding, blow molding, thermoforming, and casting. A summary of each process and its cost is displayed in figure 1.
Nylon 1,6 is a type of polyamide or nylon. Unlike most other nylons, nylon 1,6 is not a condensation polymer, but instead is formed by an acid-catalyzed synthesis from adiponitrile, formaldehyde, and water. The material was produced and studied by researchers at DuPont in the 1950s. Synthesis can be performed at room temperature in open beakers.













