Contact lenses, or simply contacts, are thin lenses placed directly on the surface of the eyes. Contact lenses are ocular prosthetic devices used by over 150 million people worldwide, and they can be worn to correct vision or for cosmetic or therapeutic reasons. In 2010, the worldwide market for contact lenses was estimated at $6.1 billion, while the US soft lens market was estimated at $2.1 billion. Multiple analysts estimated that the global market for contact lenses would reach $11.7 billion by 2015. As of 2010, the average age of contact lens wearers globally was 31 years old, and two-thirds of wearers were female.

Poly(methyl methacrylate) (PMMA) belongs to a group of materials called engineering plastics. It is a transparent thermoplastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and brands Crylux, Plexiglas, Acrylite, Astariglas, Lucite, Perclax, and Perspex, among several others. This plastic is often used in sheet form as a lightweight or shatter-resistant alternative to glass. It can also be used as a casting resin, in inks and coatings, and for many other purposes.
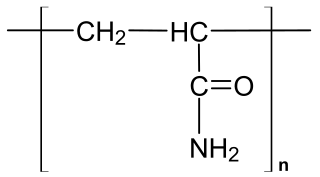
Polyacrylamide (abbreviated as PAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly for water treatment and the paper and mineral industries.

Otto Wichterle was a Czech chemist, best known for his invention of modern soft contact lenses.

A hydrogel is a crosslinked hydrophilic polymer that does not dissolve in water. They are highly absorbent yet maintain well defined structures. These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature.
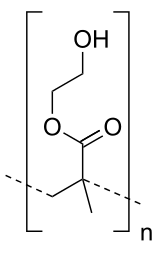
Drahoslav Lím was a Czech chemist. He invented polyhydroxyethylmethacrylate, the synthetic material used for soft contact lenses (hydrogel).

Ethylene glycol dimethylacrylate (EGDMA) is a diester formed by condensation of two equivalents of methacrylic acid and one equivalent of ethylene glycol.
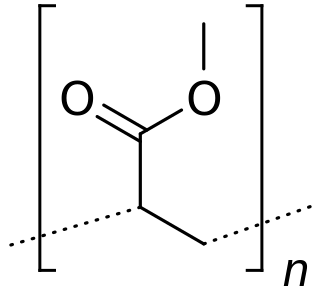
Poly(methyl acrylate) (PMA) is a hydrophobic synthetic acrylate polymer. PMA, though softer than polymethyl methacrylate (PMMA), is tough, leathery, and flexible.

A superabsorbent polymer (SAP) is a water-absorbing polymer that can absorb and retain extremely large amounts of a liquid relative to its own mass.

Hydroxyethylmethacrylate or HEMA (also known as glycol methyacrylate, GMA) is the organic compound with the formula H2C=C(CH3)CO2CH2CH2OH. It is a colorless viscous liquid that readily polymerizes. HEMA is a monomer that is used to make various polymers.
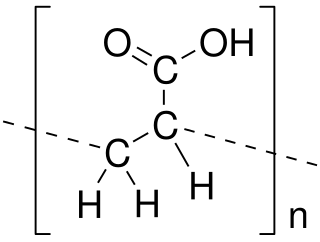
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties - acid-base and water-attracting - are the bases of many applications.
Poly(N-isopropylacrylamide) is a temperature-responsive polymer that was first synthesized in the 1950s. It can be synthesized from N-isopropylacrylamide which is commercially available. It is synthesized via free-radical polymerization and is readily functionalized making it useful in a variety of applications.

Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit a drastic and discontinuous change of their physical properties with temperature. The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, an upper or lower critical solution temperature exists, respectively.

2-Acrylamido-2-methylpropane sulfonic acid (AMPS) was a Trademark name by The Lubrizol Corporation. It is a reactive, hydrophilic, sulfonic acid acrylic monomer used to alter the chemical properties of wide variety of anionic polymers. In the 1970s, the earliest patents using this monomer were filed for acrylic fiber manufacturing. Today, there are over several thousands patents and publications involving use of AMPS in many areas including water treatment, oil field, construction chemicals, hydrogels for medical applications, personal care products, emulsion coatings, adhesives, and rheology modifiers.
Catalytic chain transfer (CCT) is a process that can be incorporated into radical polymerization to obtain greater control over the resulting products.

Jindřich Henry Kopeček was born in Strakonice, Czech Republic, as the son of Jan and Herta Zita (Krombholz) Kopeček. He is distinguished professor of pharmaceutical chemistry and distinguished professor of biomedical engineering at the University of Utah in Salt Lake City, Utah. Kopeček is also an honorary professor at Sichuan University in Chengdu, China. His research focuses on biorecognition of macromolecules, bioconjugate chemistry, drug delivery systems, self-assembled biomaterials, and drug-free macromolecular therapeutics.
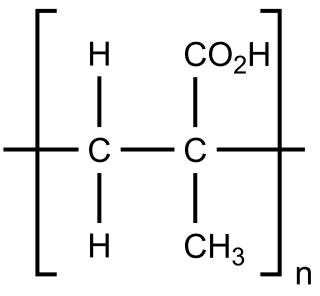
Poly(methacrylic acid) (PMAA) is a polymer made from methacrylic acid, which is a carboxylic acid. It is often available as its sodium salt, poly(methacrylic acid) sodium salt. The monomer is a viscous liquid with a pungent odour. The first polymeric form of methacrylic acid was described in 1880 by Engelhorn and Fittig. The use of high purity monomers is required for proper polymerization conditions and therefore it is necessary to remove any inhibitors by extraction or via distillation. To prevent inhibition by dissolved oxygen, monomers should be carefully degassed prior to the start of the polymerization.

Self-healing hydrogels are a specialized type of polymer hydrogel. A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. Hydrogels are synthesized from hydrophilic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. A net-like structure along with void imperfections enhance the hydrogel's ability to absorb large amounts of water via hydrogen bonding. As a result, hydrogels, self-healing alike, develop characteristic firm yet elastic mechanical properties. Self-healing refers to the spontaneous formation of new bonds when old bonds are broken within a material. The structure of the hydrogel along with electrostatic attraction forces drive new bond formation through reconstructive covalent dangling side chain or non-covalent hydrogen bonding. These flesh-like properties have motivated the research and development of self-healing hydrogels in fields such as reconstructive tissue engineering as scaffolding, as well as use in passive and preventive applications.
1-Vinylimidazole is a water-soluble basic monomer that forms quaternizable homopolymers by free-radical polymerization with a variety of vinyl and acrylic monomers. The products are functional copolymers, which are used as oil field chemicals and as cosmetic auxiliaries. 1-Vinylimidazole acts as a reactive diluent in UV lacquers, inks, and adhesives.

Pentaerythritol tetraacrylate is an organic compound. It is a tetrafunctional acrylate ester used as a monomer in the manufacture of polymers. As it is a polymerizable acrylate monomer it is nearly always supplied with a polymerisation inhibitor such as MEHQ added.