In chemistry, electron counting is a formalism for assigning a number of valence electrons to individual atoms in a molecule. It is used for classifying compounds and for explaining or predicting their electronic structure and bonding. Many rules in chemistry rely on electron-counting:

Terpyridine is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemistry.
In chemistry, a luminophore is an atom or functional group in a chemical compound that is responsible for its luminescent properties. Luminophores can be either organic or inorganic.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.

A coordination polymer is an inorganic or organometallic polymer structure containing metal cation centers linked by ligands. More formally a coordination polymer is a coordination compound with repeating coordination entities extending in 1, 2, or 3 dimensions.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.
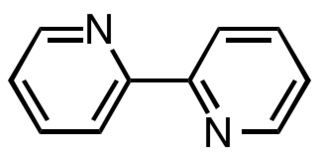
2,2′-Bipyridine is an organic compound with the formula (C5H4N)2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence.

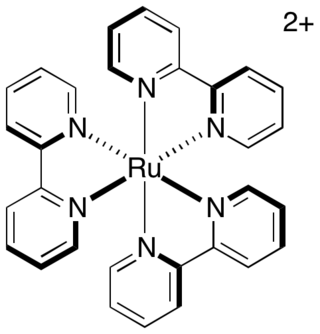
Tris(bipyridine)ruthenium(II) chloride is the chloride salt coordination complex with the formula [Ru(bpy)3]Cl2. This polypyridine complex is a red crystalline salt obtained as the hexahydrate, although all of the properties of interest are in the cation [Ru(bpy)3]2+, which has received much attention because of its distinctive optical properties. The chlorides can be replaced with other anions, such as PF6−.

A molecular sensor or chemosensor is a molecular structure that is used for sensing of an analyte to produce a detectable change or a signal. The action of a chemosensor, relies on an interaction occurring at the molecular level, usually involves the continuous monitoring of the activity of a chemical species in a given matrix such as solution, air, blood, tissue, waste effluents, drinking water, etc. The application of chemosensors is referred to as chemosensing, which is a form of molecular recognition. All chemosensors are designed to contain a signalling moiety and a recognition moiety, that is connected either directly to each other or through a some kind of connector or a spacer. The signalling is often optically based electromagnetic radiation, giving rise to changes in either the ultraviolet and visible absorption or the emission properties of the sensors. Chemosensors may also be electrochemically based. Small molecule sensors are related to chemosensors. These are traditionally, however, considered as being structurally simple molecules and reflect the need to form chelating molecules for complexing ions in analytical chemistry. Chemosensors are synthetic analogues of biosensors, the difference being that biosensors incorporate biological receptors such as antibodies, aptamers or large biopolymers.
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.

Electrochemiluminescence or electrogenerated chemiluminescence (ECL) is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited state that then emits light upon relaxation to a lower-level state. This wavelength of the emitted photon of light corresponds to the energy gap between these two states. ECL excitation can be caused by energetic electron transfer (redox) reactions of electrogenerated species. Such luminescence excitation is a form of chemiluminescence where one/all reactants are produced electrochemically on the electrodes.
Photochemical reduction of carbon dioxide harnesses solar energy to convert CO2 into higher-energy products. Environmental interest in producing artificial systems is motivated by recognition that CO2 is a greenhouse gas. The process has not been commercialized.

Charge-transfer bands are a characteristic feature of the optical spectra of many compounds. These bands are typically more intense than d–d transitions. They typically exhibit solvatochromism, consistent with shifts of electron density that would be sensitive to solvation.
Photoredox catalysis is a branch of photochemistry that uses single-electron transfer. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.
DNA-binding metallo-intercalators are positively charged, planar, polycyclic, aromatic compounds that unwind the DNA double helix and insert themselves between DNA base pairs. Metallo-intercalators insert themselves between two intact base pairs without expelling or replacing the original nitrogenous bases; the hydrogen bonds between the nitrogenous bases at the site of intercalation remain unbroken. In addition to π-stacking between the aromatic regions of the intercalator and the nitrogenous bases of DNA, intercalation is stabilized by van der Waals, hydrophobic, electrostatic, and entropic interactions. This ability to bind to specific DNA base pairs allows for potential therapeutic applications of metallo-intercalators.
Alexander von Zelewsky was a full Professor in chemistry at the University of Fribourg in Fribourg, Switzerland, from 1969 to 2006.
A transition metal phosphido complex is a coordination complex containing a phosphido ligand (R2P, where R = H, organic substituent). With two lone pairs on phosphorus, the phosphido anion (R2P−) is comparable to an amido anion (R2N−), except that the M-P distances are longer and the phosphorus atom is more sterically accessible. For these reasons, phosphido is often a bridging ligand. The -PH2 ion or ligand is also called phosphanide or phosphido ligand.

cis-Dichlorobis(bipyridine)ruthenium(II) is the coordination complex with the formula RuCl2(bipy)2, where bipy is 2,2'-bipyridine. It is a dark green diamagnetic solid that is a precursor to many other complexes of ruthenium, mainly by substitution of the two chloride ligands. The compound has been crystallized as diverse hydrates.
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine (bipy) is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties.

Dichlororuthenium tricarbonyl dimer is an organoruthenium compound with the formula [RuCl2(CO)3]2. A yellow solid, the molecule features a pair of octahedral Ru centers bridged by a pair of chloride ligands. The complex is a common starting material in ruthenium chemistry.












