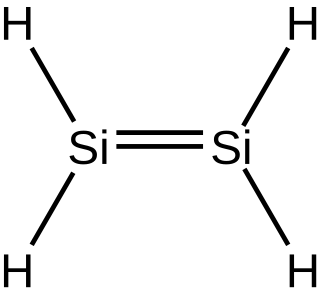
In inorganic chemistry, silenes, or disilalkenes, are silicon compounds that contain Si=Si double bonds, where the oxidation state of Si is +2. The parent molecule is disilene, Si2H4.

In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: Si−O−Si. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H[OSiH2]nOH and [OSiH2]n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones [−R2Si−O−SiR2−]n, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility.
In inorganic chemistry, chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is silicon tetrachloride.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.

Silanes are saturated chemical compounds with the empirical formula SixHy. They are hydrosilanes, a class of compounds that includes compounds with Si−H and other Si−X bonds. All contain tetrahedral silicon and terminal hydrides. They only have Si−H and Si−Si single bonds. The bond lengths are 146.0 pm for a Si−H bond and 233 pm for a Si−Si bond. The structures of the silanes are analogues of the alkanes, starting with silane, SiH4, the analogue of methane, continuing with disilane Si2H6, the analogue of ethane, etc. They are mainly of theoretical or academic interest.
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.
Silicon compounds are compounds containing the element silicon (Si). As a carbon group element, silicon often forms compounds in the +4 oxidation state, though many unusual compounds have been discovered that differ from expectations based on its valence electrons, including the silicides and some silanes. Metal silicides, silicon halides, and similar inorganic compounds can be prepared by directly reacting elemental silicon or silicon dioxide with stable metals or with halogens. Silanes, compounds of silicon and hydrogen, are often used as strong reducing agents, and can be prepared from aluminum–silicon alloys and hydrochloric acid.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.
In polymer chemistry, an inorganic polymer is a polymer with a skeletal structure that does not include carbon atoms in the backbone. Polymers containing inorganic and organic components are sometimes called hybrid polymers, and most so-called inorganic polymers are hybrid polymers. One of the best known examples is polydimethylsiloxane, otherwise known commonly as silicone rubber. Inorganic polymers offer some properties not found in organic materials including low-temperature flexibility, electrical conductivity, and nonflammability. The term inorganic polymer refers generally to one-dimensional polymers, rather than to heavily crosslinked materials such as silicate minerals. Inorganic polymers with tunable or responsive properties are sometimes called smart inorganic polymers. A special class of inorganic polymers are geopolymers, which may be anthropogenic or naturally occurring.
Dimethyldichlorosilane is a tetrahedral organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made on an industrial scale as the principal precursor to dimethylsilicone and polysilane compounds.

Methyltrichlorosilane, also known as trichloromethylsilane, is a monomer and organosilicon compound with the formula CH3SiCl3. It is a colorless liquid with a sharp odor similar to that of hydrochloric acid. As methyltrichlorosilane is a reactive compound, it is mainly used a precursor for forming various cross-linked siloxane polymers.
In organosilicon chemistry, polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula [R2Si−NR]n occur. R can be hydrogen atoms or organic substituents. If all substituents R are hydrogen atoms, the polymer is designated as perhydropolysilazane, polyperhydridosilazane, or inorganic polysilazane ([H2Si−NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R2Si−NH]n are isoelectronic with and close relatives to polysiloxanes [R2Si−O]n (silicones).
Polysilicon hydrides are polymers containing only silicon and hydrogen. They have the formula where 0.2 ≤ n ≤ 2.5 and x is the number of monomer units. The polysilicon hydrides are generally colorless or pale-yellow/ocher powders that are easily hydrolyzed and ignite readily in air. The surfaces of silicon prepared by MOCVD using silane (SiH4) consist of a polysilicon hydride.
Polysilicon halides are silicon-backbone polymeric solids. At room temperature, the polysilicon fluorides are colorless to yellow solids while the chlorides, bromides, and iodides are, respectively, yellow, amber, and red-orange. Polysilicon dihalides (perhalo-polysilenes) have the general formula (SiX2)n while the polysilicon monohalides (perhalo-polysilynes) have the formula (SiX)n, where X is F, Cl, Br, or I and n is the number of monomer units in the polymer.
In organosilicon chemistry, polysilynes are chemical compounds with the formula [RSi]n, where R can be hydrogen, or organyl. Although their name suggests a relationship to alkynes, polysilynes are a class of silicon-based random network polymers primarily composed of tetrahedral silicon atoms, each connected to one hydrogen or carbon and three Si atoms. These compounds are prepared by Wurtz coupling of alkyltrichlorosilanes :
The term preceramic polymer refers to one of various polymeric compounds, which through pyrolysis under appropriate conditions are converted to ceramic compounds, having high thermal and chemical stability. Ceramics resulting from the pyrolysis of preceramic polymers are known as polymer derived ceramics, or PDCs. Polymer derived ceramics are most often silicon based and include silicon carbide, silicon oxycarbide, silicon nitride and silicon oxynitride. Such PDCs are most commonly amorphous, lacking long-range crystalline order.
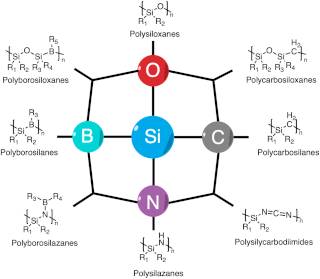
Polymer derived ceramics (PDCs) are ceramic materials formed by the pyrolysis of preceramic polymers, usually under inert atmosphere.
In organosilicon chemistry, silanes are a diverse class of charge-neutral organic compounds with the general formula SiR4. The R substituents can be any combination of organic or inorganic groups. Most silanes contain Si-C bonds, and are discussed under organosilicon compounds. Some contain Si-H bonds and are discussed under hydrosilanes.

(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2TMS. It crystallizes as the hexagonal prismatic hexamer [LiCH2TMS]6, akin to some polymorphs of methyllithium. Many adducts have been characterized including the diethyl ether complexed cubane [Li4(μ3-CH2TMS)4(Et2O)2] and [Li2(μ-CH2TMS)2(TMEDA)2].

Dodecamethylcyclohexasilane is the organosilicon compound with the formula Si6(CH3)12. It is one of the more readily prepared and easily handled polysilanes. Dodecamethylcyclohexasilane is produced by reduction of dimethyldichlorosilane with sodium-potassium alloy:









