Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.

In chemistry, a salt or ionic compound is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a neutral compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

Sodium perchlorate is an inorganic compound with the chemical formula NaClO4. It consists of sodium cations Na+ and perchlorate anions ClO−4. It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as sodium perchlorate monohydrate NaClO4·H2O. The compound is noteworthy as the most water-soluble of the common perchlorate salts.

An iodate is the polyatomic anion with the formula IO−3. It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid.

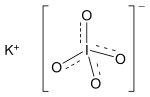
Periodate is an anion composed of iodine and oxygen. It is one of a number of oxyanions of iodine and is the highest in the series, with iodine existing in oxidation state +7. Unlike other perhalogenates, such as perchlorate, it can exist in two forms: metaperiodateIO−
4 and orthoperiodateIO5−
6. In this regard it is comparable to the tellurate ion from the adjacent group. It can combine with a number of counter ions to form periodates, which may also be regarded as the salts of periodic acid.

Iodic acid is a white water-soluble solid with the chemical formula HIO3. Its robustness contrasts with the instability of chloric acid and bromic acid. Iodic acid features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. When heated, samples dehydrate to give iodine pentoxide. On further heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.

Periodic acid is the highest oxoacid of iodine, in which the iodine exists in oxidation state +7. It can exist in two forms: orthoperiodic acid, with the chemical formula H5IO6, and metaperiodic acid, which has the formula HIO4.
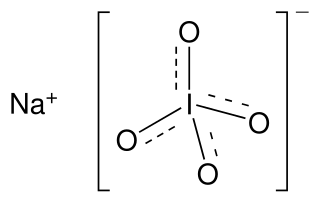
Sodium periodate is an inorganic salt, composed of a sodium cation and the periodate anion. It may also be regarded as the sodium salt of periodic acid. Like many periodates, it can exist in two different forms: sodium metaperiodate (formula NaIO4) and sodium orthoperiodate (normally Na2H3IO6, but sometimes the fully reacted salt Na5IO6). Both salts are useful oxidising agents.

Potassium cyanate is an inorganic compound with the formula KOCN. It is a colourless solid. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.

Phosphotungstic acid (PTA) or tungstophosphoric acid (TPA), is a heteropoly acid with the chemical formula H3PW12O40]. It forms hydrates H3[PW12O40]·nH2O. It is normally isolated as the n = 24 hydrate but can be desiccated to the hexahydrate (n = 6). EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly yellow-green crystals, with melting point 89 °C (24 H2O hydrate). It is odorless and soluble in water (200 g/100 ml). It is not especially toxic, but is a mild acidic irritant. The compound is known by a variety of names and acronyms (see 'other names' section of infobox).
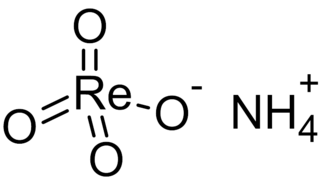
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. It is the most common form in which rhenium is traded. It is a white salt; soluble in ethanol and water, and mildly soluble in NH4Cl. It was first described soon after the discovery of rhenium.

Sodium iodate (NaIO3) is the sodium salt of iodic acid. Sodium iodate is an oxidizing agent. It has several uses.
Iodine compounds are compounds containing the element iodine. Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but it is much less reactive than the other halogens. For example, while chlorine gas will halogenate carbon monoxide, nitric oxide, and sulfur dioxide, iodine will not do so. Furthermore, iodination of metals tends to result in lower oxidation states than chlorination or bromination; for example, rhenium metal reacts with chlorine to form rhenium hexachloride, but with bromine it forms only rhenium pentabromide and iodine can achieve only rhenium tetraiodide. By the same token, however, since iodine has the lowest ionisation energy among the halogens and is the most easily oxidised of them, it has a more significant cationic chemistry and its higher oxidation states are rather more stable than those of bromine and chlorine, for example in iodine heptafluoride.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.
Lead(II) iodate is an inorganic compound with the molecular formula Pb(IO3)2. It is naturally found as heavy white powder.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.
The periodatonickelates are a series of anions and salts of nickel complexed to the periodate anion. The most important of these salts are the diperiodatonickelates, in which nickel exhibits the +4 oxidation state: these are powerful oxidising agents, capable of oxidising bromate to perbromate.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.

Ammonium iodate is an inorganic salt which is sparingly soluble in cold, and moderately soluble in hot water, like all iodate salts, it is a strong oxidizer.
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions [SO3]2− [SO4]2−. These compounds were discovered in the 1980s as calcium and rare earth element salts. Minerals in this class were later discovered. Minerals may have sulfite as an essential component, or have it substituted for another anion as in alloriite. The related ions [O3SOSO2]2− and [(O2SO)2SO2]2− may be produced in a reaction between sulfur dioxide and sulfate and exist in the solid form as tetramethyl ammonium salts. They have a significant partial pressure of sulfur dioxide.