Piezoelectricity is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure and latent heat. It is derived from Ancient Greek πιέζω (piézō) 'to squeeze or press', and ἤλεκτρον (ḗlektron) 'amber'. The German form of the word (Piezoelektricität) was coined in 1881 by the German physicist Wilhelm Gottlieb Hankel; the English word was coined in 1883.

Potassium ferrocyanide is the inorganic compound with formula K4[Fe(CN)6]·3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.

Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid.

Potassium chloride is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners, and in food processing, where it may be known as E number additive E508.

Potassium tartrate, dipotassium tartrate or argol has formula K2C4H4O6. It is the potassium salt of tartaric acid. It is often confused with potassium bitartrate, also known as cream of tartar. As a food additive, it shares the E number E336 with potassium bitartrate.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Potassium bicarbonate (IUPAC name: potassium hydrogencarbonate, also known as potassium acid carbonate) is the inorganic compound with the chemical formula KHCO3. It is a white solid.

Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used

Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride. Its chemical formula is (C2H2F2)n.

In organic chemistry, Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone functional groups, and as a test for reducing sugars and non-reducing sugars, supplementary to the Tollens' reagent test. The test was developed by German chemist Hermann von Fehling in 1849.

Potassium bitartrate, also known as potassium hydrogen tartrate, with formula KC4H5O6, is a chemical compound with a number of uses. It is the potassium acid salt of tartaric acid (a carboxylic acid). In cooking, it is known as cream of tartar.

Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF. A hygroscopic white salt, caesium fluoride is used in the synthesis of organic compounds as a source of the fluoride anion. The compound is noteworthy from the pedagogical perspective as caesium also has the highest electropositivity of all commonly available elements and fluorine has the highest electronegativity.

A tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. The formula of the tartrate dianion is O−OC-CH(OH)-CH(OH)-COO− or C4H4O62−.
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic mixture into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution.
The Lemieux–Johnson or Malaprade–Lemieux–Johnson oxidation is a chemical reaction in which an olefin undergoes oxidative cleavage to form two aldehyde or ketone units. The reaction is named after its inventors, Raymond Urgel Lemieux and William Summer Johnson, who published it in 1956. The reaction proceeds in a two step manner, beginning with dihydroxylation of the alkene by osmium tetroxide, followed by a Malaprade reaction to cleave the diol using periodate. Excess periodate is used to regenerate the osmium tetroxide, allowing it to be used in catalytic amounts. The Lemieux–Johnson reaction ceases at the aldehyde stage of oxidation and therefore produces the same results as ozonolysis.

Ammonium dihydrogen phosphate (ADP), also known as monoammonium phosphate (MAP) is a chemical compound with the chemical formula (NH4)(H2PO4). ADP is a major ingredient of agricultural fertilizers and dry chemical fire extinguishers. It also has significant uses in optics and electronics.

Monosodium tartrate or sodium bitartrate is a sodium acid salt of tartaric acid. As a food additive it is used as an acidity regulator and is known by the E number E335. As an analytical reagent, it can be used in a test for ammonium cation which gives a white precipitate.
Sodium hypobromite is an inorganic compound with the chemical formula NaOBr. It is a sodium salt of hypobromous acid. It consists of sodium cations Na+ and hypobromite anions −OBr. It is usually obtained as the pentahydrate, so the compound that is usually called sodium hypobromite actually has the formula NaOBr·5H2O. It is a yellow-orange solid that is soluble in water. It adopts a monoclinic crystal structure with a Br–O bond length of 1.820 Å. It is the bromine analogue of sodium hypochlorite, the active ingredient in common bleach. In practice the salt is usually encountered as an aqueous solution.

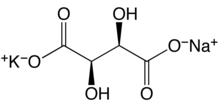
Sodium ammonium tartrate (NAT) is an organic compound with the formula Na(NH4)[O2CCH(OH)CH(OH)CO2]. The salt is derived from tartaric acid by neutralizing with ammonia and with sodium hydroxide. Louis Pasteur obtained enantiopure crystals of the tetrahydrate of NAT, via the process of spontaneous resolution. His discovery led to increased study of optical activity, which eventually was shown to have broad implications. Many modification of this salt have been investigated by X-ray crystallography, including the racemate, which crystallizes as the monohydrate.