In chemistry, an oxonium ion is any cation containing an oxygen atom that has three bonds and 1+ formal charge. The simplest oxonium ion is the hydronium ion.
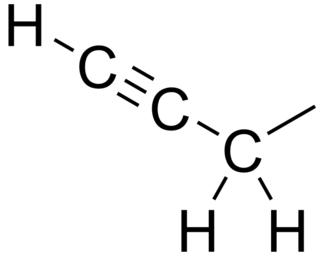
In organic chemistry, the propargyl group is a functional group of 2-propynyl with the structure CH≡C−CH2−. It is an alkyl group derived from propyne.
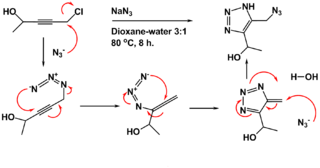
The Banert cascade is an organic reaction in which an NH-1,2,3-triazole is prepared from a propargyl halide or sulfate and sodium azide in a dioxane- water mixture at elevated temperatures. It is named after Klaus Banert, who first reported the process in 1989. This cascade reaction is unusual because it consists of two consecutive rearrangement reactions.
The Larock indole synthesis is a heteroannulation reaction that uses palladium as a catalyst to synthesize indoles from an ortho-iodoaniline and a disubstituted alkyne. It is also known as Larock heteroannulation. The reaction is extremely versatile and can be used to produce varying types of indoles. Larock indole synthesis was first proposed by Richard C. Larock in 1991 at Iowa State University.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

Propiolic acid is the organic compound with the formula HC2CO2H. It is the simplest acetylenic carboxylic acid. It is a colourless liquid that crystallises to give silky crystals. Near its boiling point, it decomposes.

Organocadmium chemistry describes the physical properties, synthesis, reactions, and use of organocadmium compounds, which are organometallic compounds containing a carbon to cadmium chemical bond. Cadmium shares group 12 with zinc and mercury and their corresponding chemistries have much in common. The synthetic utility of organocadmium compounds is limited.
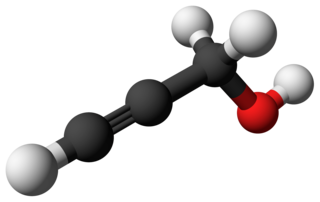
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents.
In organic chemistry, the Paal–Knorr synthesis is a reaction used to synthesize substituted furans, pyrroles, or thiophenes from 1,4-diketones. It is a synthetically valuable method for obtaining substituted furans and pyrroles, which are common structural components of many natural products. It was initially reported independently by German chemists Carl Paal and Ludwig Knorr in 1884 as a method for the preparation of furans, and has been adapted for pyrroles and thiophenes. Although the Paal–Knorr synthesis has seen widespread use, the mechanism wasn't fully understood until it was elucidated by V. Amarnath et al. in the 1990s.

The Whiting reaction is an organic reaction converting a propargyl diol into a diene using lithium aluminium hydride.
The Favorskii reaction is an organic chemistry reaction between an alkyne and a carbonyl group, under basic conditions. The reaction was discovered in the early 1900s by the Russian chemist Alexei Yevgrafovich Favorskii.
The Schotten–Baumann reaction is a method to synthesize amides from amines and acid chlorides:

Organosilver chemistry is the study of organometallic compounds containing a carbon to silver chemical bond. The theme is less developed than organocopper chemistry.

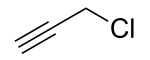
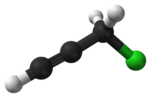
Propargyl bromide, also known as 3-bromo-prop-1-yne, is an organic compound with the chemical formula HC≡CCH2Br. A colorless liquid, it is a halogenated organic compound consisting of propyne with a bromine substituent on the methyl group. It has a lachrymatory effect, like related compounds. The compound is used as a reagent in organic synthesis.

Propiolaldehyde is an organic compound with molecular formula HC2CHO. It is the simplest chemical compound containing both alkyne and aldehyde functional groups. It is a colorless liquid with explosive properties.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.

3-Dimethylaminoacrolein is an organic compound with the formula Me2NC(H)=CHCHO. It is a pale yellow water-soluble liquid. The compound has a number of useful and unusual properties, e.g. it "causes a reversal of the hypnotic effect of morphine in mice" and has a "stimulating effect in humans".
In chemistry, decomplexation refers to the removal of a ligand from a coordination complex. Decomplexation is of particular interest when the ligand has been synthesized within the coordination sphere of the metal, as is often the case in organometallic chemistry.
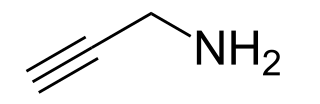
Propargylamine is an organic compound with the formula HC≡CCH2NH2. It is a colorless, odorless liquid that is used as a precursor to other compounds. Propargyl amines are produced by reactions of amines with propargyl halides.

1,4-Pentadiyne (penta-1,4-diyne) is a chemical compound belonging to the alkynes. The compound is the structural isomer to 1,3-pentadiyne.