
In chemistry, alcohol is an organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic beverages. An important class of alcohols, of which methanol and ethanol are the simplest members, includes all compounds for which the general formula is CnH2n+1OH. Simple monoalcohols that are the subject of this article include primary (RCH2OH), secondary (R2CHOH) and tertiary (R3COH) alcohols.
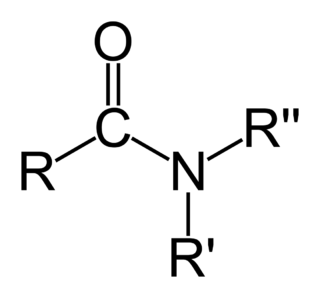
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula RC(=O)NR′R″, where R, R′, and R″ represent organic groups or hydrogen atoms.. The amide group is called a peptide bond when it is part of the main chain of a protein, and isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid RC(=O)OH with the hydroxyl group –OH replaced by an amine group –NR′R″; or, equivalently, an acyl (alkanoyl) group RC(=O)– joined to an amine group.
Catalysis is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst, which is not consumed in the catalyzed reaction and can continue to act repeatedly. Because of this, only very small amounts of catalyst are required to alter the reaction rate in most cases.

A carboxylic acid is an organic compound that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R–COOH, with R referring to the alkyl group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In chemistry, an ester is a chemical compound derived from an acid in which at least one –OH (hydroxyl) group is replaced by an –O–alkyl (alkoxy) group. Usually, esters are derived from a carboxylic acid and an alcohol. Glycerides, which are fatty acid esters of glycerol, are important esters in biology, being one of the main classes of lipids, and making up the bulk of animal fats and vegetable oils. Esters with low molecular weight are commonly used as fragrances and found in essential oils and pheromones. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties, while polyesters are important plastics, with monomers linked by ester moieties. Esters usually have a sweet smell and are considered high-quality solvents for a broad array of plastics, plasticizers, resins, and lacquers. They are also one of the largest classes of synthetic lubricants on the commercial market.

In organic chemistry, functional groups are specific substituents or moieties within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of. This allows for systematic prediction of chemical reactions and behavior of chemical compounds and design of chemical syntheses. Furthermore, the reactivity of a functional group can be modified by other functional groups nearby. In organic synthesis, functional group interconversion is one of the basic types of transformations.
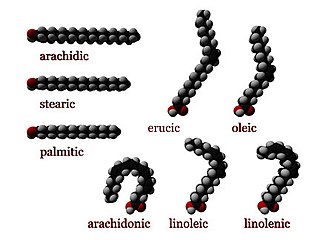
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually not found in organisms, but instead as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and they are important structural components for cells.
A structural isomer, or constitutional isomer, is a type of isomer in which molecules with the same molecular formula have different bonding patterns and atomic organization, as opposed to stereoisomers, in which molecular bonds are always in the same order and only spatial arrangement differs. There are multiple synonyms for structural isomers.

In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

Fischer esterification or Fischer–Speier esterification is a special type of esterification by refluxing a carboxylic acid and an alcohol in the presence of an acid catalyst. The reaction was first described by Emil Fischer and Arthur Speier in 1895. Most carboxylic acids are suitable for the reaction, but the alcohol should generally be primary or secondary. Tertiary alcohols are prone to elimination. Contrary to common misconception found in organic chemistry textbooks, phenols can also be esterified to give good to near quantitative yield of products. Commonly used catalysts for a Fischer esterification include sulfuric acid, tosylic acid, and Lewis acids such as scandium(III) triflate. For more valuable or sensitive substrates other, milder procedures such as Steglich esterification are used. The reaction is often carried out without a solvent or in a non-polar solvent to facilitate the Dean-Stark method. Typical reaction times vary from 1–10 hours at temperatures of 60-110 °C.

Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. The term "unsaturated" means more hydrogen atoms may be added to the hydrocarbon to make it saturated. The configuration of an unsaturated carbons include straight chain, such as alkenes and alkynes, as well as branched chains and aromatic compounds.
A non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/mol (1000–5000 calories per 6.02 × 1023 molecules). Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects.

Propyl acetate, also known as propyl ethanoate, is a chemical compound used as a solvent and an example of an ester. This clear, colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fragrances and as a flavor additive. It is formed by the esterification of acetic acid and 1-propanol, often via Fischer–Speier esterification, with sulfuric acid as a catalyst and water produced as a byproduct.

1-Propanol is a primary alcohol with the formula CH
3CH
2CH
2OH. This colorless liquid is also known as propan-1-ol, 1-propyl alcohol, n-propyl alcohol, and n-propanol. It is an isomer of 2-propanol. It is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent.

A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water. In contrast, hydrophobes are not attracted to water and may seem to be repelled by it.

Dimethylol ethyleneurea is an organic compound derived from formaldehyde and urea. It is a colourless solid that is used for treating cellulose-based heavy fabrics to inhibit wrinkle formation. Dimethylol ethylene urea (DMEU) bonds with the hydroxyl groups present in long cellulose chains and prevents the formation hydrogen bonding between the chains, the primary cause of wrinkling. This treatment produces permanently wrinkle-resistant fabrics and is different from the effects achieved from using fabric softeners.
















