Unsymmetrical dimethylhydrazine (abbreviated as UDMH; also known as 1,1-dimethylhydrazine, heptyl or Geptil) is a chemical compound with the formula H2NN(CH3)2 that is primarily used as a rocket propellant. At room temperature, UDMH is a colorless liquid, with a sharp, fishy, ammonia-like smell typical of organic amines. Samples turn yellowish on exposure to air and absorb oxygen and carbon dioxide. It is miscible with water, ethanol, and kerosene. At concentrations between 2.5% and 95% in air, its vapors are flammable. It is not sensitive to shock.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.

1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula CH3Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It is a recognized ozone-depleting chemical. It was used extensively as a pesticide until being phased out by most countries in the early 2000s. From a chemistry perspective, it is one of the halomethanes.
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH3Cl. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant. Most chloromethane is biogenic.
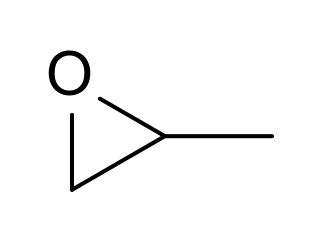
Propylene oxide is an acutely toxic and carcinogenic organic compound with the molecular formula C3H6O. This colourless volatile liquid with an odour similar to ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.

Ethyl acetate is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.

Toluene diisocyanate (TDI) is an organic compound with the formula CH3C6H3(NCO)2. Two of the six possible isomers are commercially important: 2,4-TDI (CAS: 584-84-9) and 2,6-TDI (CAS: 91-08-7). 2,4-TDI is produced in the pure state, but TDI is often marketed as 80/20 and 65/35 mixtures of the 2,4 and 2,6 isomers respectively. It is produced on a large scale, accounting for 34.1% of the global isocyanate market in 2000, second only to MDI. Approximately 1.4 billion kilograms were produced in 2000. All isomers of TDI are colorless, although commercial samples can appear yellow.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.
Aziridine is an organic compound consisting of the three-membered heterocycle C2H5N. It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivatives, also referred to as aziridines, are of broader interest in medicinal chemistry.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

Cumene (isopropylbenzene) is an organic compound that contains a benzene ring with an isopropyl substituent. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C. Nearly all the cumene that is produced as a pure compound on an industrial scale is converted to cumene hydroperoxide, which is an intermediate in the synthesis of other industrially important chemicals, primarily phenol and acetone.

Isophorone diisocyanate (IPDI) is an organic compound in the class known as isocyanates. More specifically, it is an aliphatic diisocyanate. It is produced in relatively small quantities, accounting for only 3.4% of the global diisocyanate market in the year 2000. Aliphatic diisocyanates are used, not in the production of polyurethane foam, but in special applications, such as enamel coatings which are resistant to abrasion and degradation from ultraviolet light. These properties are particularly desirable in, for instance, the exterior paint applied to aircraft.

Propylene glycol dinitrate (PGDN, 1,2-propylene glycol dinitrate, or 1,2-propanediol dinitrate) is an organic chemical, an ester of nitric acid and propylene glycol. It is structurally similar to nitroglycerin, except that it has one fewer nitrate group. It is a characteristically and unpleasantly smelling colorless liquid, which decomposes at 121 °C, below its boiling point. It is flammable and explosive. It is shock-sensitive and burns with a clean flame producing water vapor, carbon monoxide, and nitrogen gas.

Hexachloroethane (perchloroethane) is an organochlorine compound with the chemical formula (CCl3)2. It is a white or colorless solid at room temperature with a camphor-like odor. It has been used by the military in smoke compositions, such as base-eject smoke munitions.

2-Aminopyridine is an organic compound with the formula H2NC5H4N. It is one of three isomeric aminopyridines. It is a colourless solid that is used in the production of the drugs piroxicam, sulfapyridine, tenoxicam, and tripelennamine. It is produced by the reaction of sodium amide with pyridine, the Chichibabin reaction.

1,2-Dichloropropane is an organic compound classified as a chlorocarbon. It is a colorless, flammable liquid with a sweet odor. it is obtained as a byproduct of the production of epichlorohydrin, which is produced on a large scale.
4,4′-Methylenedianiline (MDA) is an organic compound with the formula CH2(C6H4NH2)2. It is a colorless solid, although commercial samples can appear yellow or brown. It is produced on an industrial scale, mainly as a precursor to polyurethanes.



















