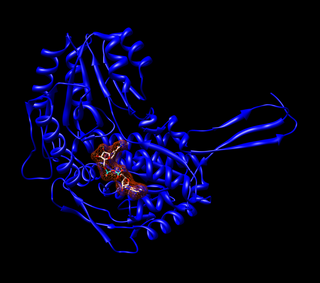
Acetaldehyde dehydrogenases are dehydrogenase enzymes which catalyze the conversion of acetaldehyde into acetyl-CoA. This can be summarized as follows:

Glutamate dehydrogenase is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typically, the α-ketoglutarate to glutamate reaction does not occur in mammals, as glutamate dehydrogenase equilibrium favours the production of ammonia and α-ketoglutarate. Glutamate dehydrogenase also has a very low affinity for ammonia, and therefore toxic levels of ammonia would have to be present in the body for the reverse reaction to proceed. However, in brain, the NAD+/NADH ratio in brain mitochondria encourages oxidative deamination. In bacteria, the ammonia is assimilated to amino acids via glutamate and aminotransferases. In plants, the enzyme can work in either direction depending on environment and stress. Transgenic plants expressing microbial GLDHs are improved in tolerance to herbicide, water deficit, and pathogen infections. They are more nutritionally valuable.

Glycogen phosphorylase is one of the phosphorylase enzymes. Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects.

Trimethylglycine is an amino acid derivative that occurs in plants. Trimethylglycine was the first betaine discovered; originally it was simply called betaine because, in the 19th century, it was discovered in sugar beets.
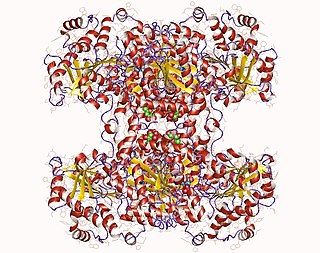
Glycogen synthase is a key enzyme in glycogenesis, the conversion of glucose into glycogen. It is a glycosyltransferase that catalyses the reaction of UDP-glucose and n to yield UDP and n+1.
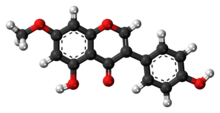
Aldehyde dehydrogenases are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes to carboxylic acids. The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes.

Calciseptine (CaS) is a natural neurotoxin isolated from the black mamba Dendroaspis p. polylepis venom. This toxin consists of 60 amino acids with four disulfide bonds. Calciseptine specifically blocks L-type calcium channels, but not other voltage-dependent Ca2+ channels such as N-type and T-type channels.

Myophosphorylase or glycogen phosphorylase, muscle associated (PYGM) is the muscle isoform of the enzyme glycogen phosphorylase and is encoded by the PYGM gene. This enzyme helps break down glycogen into glucose-1-phosphate, so it can be used within the muscle cell. Mutations in this gene are associated with McArdle disease, a glycogen storage disease of muscle.

Aldehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ALDH2 gene located on chromosome 12. ALDH2 belongs to the aldehyde dehydrogenase family of enzymes. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. ALDH2 has a low Km for acetaldehyde, and is localized in mitochondrial matrix. The other liver isozyme, ALDH1, localizes to the cytosol.

Xanthine dehydrogenase, also known as XDH, is a protein that, in humans, is encoded by the XDH gene.

In enzymology, a retinal dehydrogenase, also known as retinaldehyde dehydrogenase (RALDH), catalyzes the chemical reaction converting retinal to retinoic acid. This enzyme belongs to the family of oxidoreductases, specifically the class acting on aldehyde or oxo- donor groups with NAD+ or NADP+ as acceptor groups, the systematic name being retinal:NAD+ oxidoreductase. This enzyme participates in retinol metabolism. The general scheme for the reaction catalyzed by this enzyme is:

Aldehyde dehydrogenase, dimeric NADP-preferring is an enzyme that in humans is encoded by the ALDH3A1 gene.

Aldo-keto reductase family 1 member C4, also known as 3α-Hydroxysteroid dehydrogenase type 1 (3α-HSD1), is an enzyme that in humans is encoded by the AKR1C4 gene. It is known to be necessary for the synthesis of the endogenous neurosteroids allopregnanolone, tetrahydrodeoxycorticosterone, and 3α-androstanediol. It is also known to catalyze the reversible conversion of 3α-androstanediol (5α-androstane-3α,17β-diol) to dihydrotestosterone and vice versa.

Alcohol dehydrogenase 1A is an enzyme that in humans is encoded by the ADH1A gene.

10-formyltetrahydrofolate dehydrogenase is an enzyme that in humans is encoded by the ALDH1L1 gene.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

5α-Dihydroprogesterone is an endogenous progestogen and neurosteroid that is synthesized from progesterone. It is also an intermediate in the synthesis of allopregnanolone and isopregnanolone from progesterone.

Genistin is an isoflavone found in a number of dietary plants like soy and kudzu. It was first isolated in 1931 from the 90% methanol extract of a soybean meal, when it was found that hydrolysis with hydrochloric acid produced 1 mole each of genistein and glucose. Chemically it is the 7-O-beta-D-glucoside form of genistein and is the predominant form of the isoflavone naturally occurring in plants. In fact, studies in the 1970s revealed that 99% of the isoflavonoid compounds in soy are present as their glucosides. The glucosides are converted by digestive enzymes in the digestive system to exert their biological effects. Genistin is also converted to a more familiar genistein, thus, the biological activities including antiatherosclerotic, estrogenic and anticancer effects are analogous.

Alcohol intolerance is due to a genetic polymorphism of the aldehyde dehydrogenase enzyme, which is responsible for the metabolism of acetaldehyde. This polymorphism is most often reported in patients of East Asian descent. Alcohol intolerance may also be an associated side effect of certain drugs such as disulfiram, metronidazole, or nilutamide. Skin flushing and nasal congestion are the most common symptoms of intolerance after alcohol ingestion. It may also be characterized as intolerance causing hangover symptoms similar to the "disulfiram-like reaction" of aldehyde dehydrogenase deficiency or chronic fatigue syndrome. Severe pain after drinking alcohol may indicate a more serious underlying condition.

The pharmacology of ethanol involves both pharmacodynamics and pharmacokinetics. In the body, ethanol primarily affects the central nervous system, acting as a depressant and causing sedation, relaxation, and decreased anxiety. The complete list of mechanisms remains an area of research, but ethanol has been shown to affect ligand-gated ion channels, particularly the GABAA receptor.