Related Research Articles
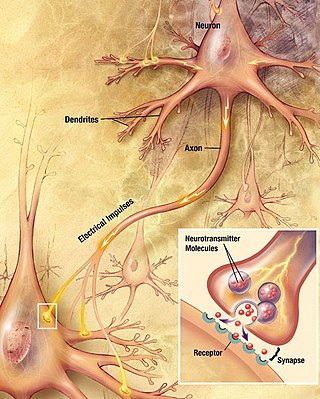
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous system. They are crucial to the biological computations that underlie perception and thought. They allow the nervous system to connect to and control other systems of the body.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. IPSP were first investigated in motorneurons by David P. C. Lloyd, John Eccles and Rodolfo Llinás in the 1950s and 1960s. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell to cell signalling. Inhibitory presynaptic neurons release neurotransmitters that then bind to the postsynaptic receptors; this induces a change in the permeability of the postsynaptic neuronal membrane to particular ions. An electric current that changes the postsynaptic membrane potential to create a more negative postsynaptic potential is generated, i.e. the postsynaptic membrane potential becomes more negative than the resting membrane potential, and this is called hyperpolarisation. To generate an action potential, the postsynaptic membrane must depolarize—the membrane potential must reach a voltage threshold more positive than the resting membrane potential. Therefore, hyperpolarisation of the postsynaptic membrane makes it less likely for depolarisation to sufficiently occur to generate an action potential in the postsynaptic neurone.

In neuroscience, an excitatory postsynaptic potential (EPSP) is a postsynaptic potential that makes the postsynaptic neuron more likely to fire an action potential. This temporary depolarization of postsynaptic membrane potential, caused by the flow of positively charged ions into the postsynaptic cell, is a result of opening ligand-gated ion channels. These are the opposite of inhibitory postsynaptic potentials (IPSPs), which usually result from the flow of negative ions into the cell or positive ions out of the cell. EPSPs can also result from a decrease in outgoing positive charges, while IPSPs are sometimes caused by an increase in positive charge outflow. The flow of ions that causes an EPSP is an excitatory postsynaptic current (EPSC).
In neuroscience, a silent synapse is an excitatory glutamatergic synapse whose postsynaptic membrane contains NMDA-type glutamate receptors but no AMPA-type glutamate receptors. These synapses are named "silent" because normal AMPA receptor-mediated signaling is not present, rendering the synapse inactive under typical conditions. Silent synapses are typically considered to be immature glutamatergic synapses. As the brain matures, the relative number of silent synapses decreases. However, recent research on hippocampal silent synapses shows that while they may indeed be a developmental landmark in the formation of a synapse, that synapses can be "silenced" by activity, even once they have acquired AMPA receptors. Thus, silence may be a state that synapses can visit many times during their lifetimes.
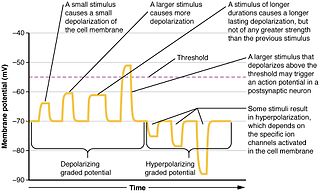
Graded potentials are changes in membrane potential that vary in size, as opposed to being all-or-none. They include diverse potentials such as receptor potentials, electrotonic potentials, subthreshold membrane potential oscillations, slow-wave potential, pacemaker potentials, and synaptic potentials, which scale with the magnitude of the stimulus. They arise from the summation of the individual actions of ligand-gated ion channel proteins, and decrease over time and space. They do not typically involve voltage-gated sodium and potassium channels. These impulses are incremental and may be excitatory or inhibitory. They occur at the postsynaptic dendrite in response to presynaptic neuron firing and release of neurotransmitter, or may occur in skeletal, smooth, or cardiac muscle in response to nerve input. The magnitude of a graded potential is determined by the strength of the stimulus.

An excitatory synapse is a synapse in which an action potential in a presynaptic neuron increases the probability of an action potential occurring in a postsynaptic cell. Neurons form networks through which nerve impulses travel, each neuron often making numerous connections with other cells. These electrical signals may be excitatory or inhibitory, and, if the total of excitatory influences exceeds that of the inhibitory influences, the neuron will generate a new action potential at its axon hillock, thus transmitting the information to yet another cell.
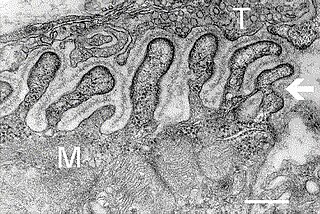
A neuromuscular junction is a chemical synapse between a motor neuron and a muscle fiber.
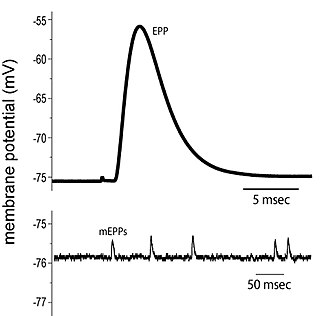
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic.
Postsynaptic potentials are changes in the membrane potential of the postsynaptic terminal of a chemical synapse. Postsynaptic potentials are graded potentials, and should not be confused with action potentials although their function is to initiate or inhibit action potentials. They are caused by the presynaptic neuron releasing neurotransmitters from the terminal bouton at the end of an axon into the synaptic cleft. The neurotransmitters bind to receptors on the postsynaptic terminal, which may be a neuron or a muscle cell in the case of a neuromuscular junction. These are collectively referred to as postsynaptic receptors, since they are on the membrane of the postsynaptic cell.
Schaffer collaterals are axon collaterals given off by CA3 pyramidal cells in the hippocampus. These collaterals project to area CA1 of the hippocampus and are an integral part of memory formation and the emotional network of the Papez circuit, and of the hippocampal trisynaptic loop. It is one of the most studied synapses in the world and named after the Hungarian anatomist-neurologist Károly Schaffer.

Neurotransmission is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron, and bind to and react with the receptors on the dendrites of another neuron a short distance away. A similar process occurs in retrograde neurotransmission, where the dendrites of the postsynaptic neuron release retrograde neurotransmitters that signal through receptors that are located on the axon terminal of the presynaptic neuron, mainly at GABAergic and glutamatergic synapses.

In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another neuron or to the target effector cell.

Synaptic potential refers to the potential difference across the postsynaptic membrane that results from the action of neurotransmitters at a neuronal synapse. In other words, it is the “incoming” signal that a neuron receives. There are two forms of synaptic potential: excitatory and inhibitory. The type of potential produced depends on both the postsynaptic receptor, more specifically the changes in conductance of ion channels in the post synaptic membrane, and the nature of the released neurotransmitter. Excitatory post-synaptic potentials (EPSPs) depolarize the membrane and move the potential closer to the threshold for an action potential to be generated. Inhibitory postsynaptic potentials (IPSPs) hyperpolarize the membrane and move the potential farther away from the threshold, decreasing the likelihood of an action potential occurring. The Excitatory Post Synaptic potential is most likely going to be carried out by the neurotransmitters glutamate and acetylcholine, while the Inhibitory post synaptic potential will most likely be carried out by the neurotransmitters gamma-aminobutyric acid (GABA) and glycine. In order to depolarize a neuron enough to cause an action potential, there must be enough EPSPs to both depolarize the postsynaptic membrane from its resting membrane potential to its threshold and counterbalance the concurrent IPSPs that hyperpolarize the membrane. As an example, consider a neuron with a resting membrane potential of -70 mV (millivolts) and a threshold of -50 mV. It will need to be raised 20 mV in order to pass the threshold and fire an action potential. The neuron will account for all the many incoming excitatory and inhibitory signals via summative neural integration, and if the result is an increase of 20 mV or more, an action potential will occur.

The Calyx of Held is a particularly large synapse in the mammalian auditory central nervous system, so named after Hans Held who first described it in his 1893 article Die centrale Gehörleitung because of its resemblance to the calyx of a flower. Globular bushy cells in the anteroventral cochlear nucleus (AVCN) send axons to the contralateral medial nucleus of the trapezoid body (MNTB), where they synapse via these calyces on MNTB principal cells. These principal cells then project to the ipsilateral lateral superior olive (LSO), where they inhibit postsynaptic neurons and provide a basis for interaural level detection (ILD), required for high frequency sound localization. This synapse has been described as the largest in the brain.

Summation, which includes both spatial summation and temporal summation, is the process that determines whether or not an action potential will be generated by the combined effects of excitatory and inhibitory signals, both from multiple simultaneous inputs, and from repeated inputs. Depending on the sum total of many individual inputs, summation may or may not reach the threshold voltage to trigger an action potential.

Axon terminals are distal terminations of the telodendria (branches) of an axon. An axon, also called a nerve fiber, is a long, slender projection of a nerve cell, or neuron, that conducts electrical impulses called action potentials away from the neuron's cell body, or soma, in order to transmit those impulses to other neurons, muscle cells or glands.
Cellular neuroscience is a branch of neuroscience concerned with the study of neurons at a cellular level. This includes morphology and physiological properties of single neurons. Several techniques such as intracellular recording, patch-clamp, and voltage-clamp technique, pharmacology, confocal imaging, molecular biology, two photon laser scanning microscopy and Ca2+ imaging have been used to study activity at the cellular level. Cellular neuroscience examines the various types of neurons, the functions of different neurons, the influence of neurons upon each other, and how neurons work together.

The active zone or synaptic active zone is a term first used by Couteaux and Pecot-Dechavassinein in 1970 to define the site of neurotransmitter release. Two neurons make near contact through structures called synapses allowing them to communicate with each other. As shown in the adjacent diagram, a synapse consists of the presynaptic bouton of one neuron which stores vesicles containing neurotransmitter, and a second, postsynaptic neuron which bears receptors for the neurotransmitter, together with a gap between the two called the synaptic cleft. When an action potential reaches the presynaptic bouton, the contents of the vesicles are released into the synaptic cleft and the released neurotransmitter travels across the cleft to the postsynaptic neuron and activates the receptors on the postsynaptic membrane.

Synaptic fatigue, or short-term synaptic depression, is an activity-dependent form of short term synaptic plasticity that results in the temporary inability of neurons to fire and therefore transmit an input signal. It is thought to be a form of negative feedback in order to physiologically control particular forms of nervous system activity.
References
- 1 2 3 4 5 Purves, Dale; Augustine, George; Fitzpatrick, David; Hall, William; LaMantia, Anthony-Samuel; White, Leonard; Mooney, Richard; Platt, Michael (eds.). Neuroscience (Fifth ed.). Sunderland, Massachusetts: Sinaur Associates, Inc.
- ↑ Schneggenburger, Ralf; Meyer, Alexander; Neher, Erwin (June 1999). "Released fraction and total size of a pool of immediately available transmitte quanta at a calyx synapse". Neuron. 23 (2): 399–409. doi:10.1016/s0896-6273(00)80789-8. hdl: 11858/00-001M-0000-0012-FB9B-0 . PMID 10399944. S2CID 13005993.
- ↑ Urbano, Francesco; Piedras-Renteria, Erika; Jun, Kisun; Shin, Hee-Sup; Uchitel, Osvaldo; Tsien, Richard (2003-03-18). "Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels". Proceedings of the National Academy of Sciences of the United States of America. 100 (6): 3491–3496. Bibcode:2003PNAS..100.3491U. doi: 10.1073/pnas.0437991100 . JSTOR 3139387. PMC 152320 . PMID 12624181.
- ↑ Minneci, Federico; Kanichay, Roby; Silver, R. Angus (30 March 2012). "Estimation of the time course of neurotransmitter release at central synapses from the first latency of postsynaptic currents". Journal of Neuroscience Methods. 205 (1): 49–64. doi:10.1016/j.jneumeth.2011.12.015. PMC 3314961 . PMID 22226741.
- ↑ Clayton, Emma; Anggono, Victor; Smillie, Karen; Chau, Ngoc; Robinson, Phillip; Cousin, Michael (June 17, 2009). "The phospho-dependent dynamin–syndapin interaction". The Journal of Neuroscience. 29 (24): 7706–7717. doi:10.1523/jneurosci.1976-09.2009. PMC 2713864 . PMID 19535582.