Related Research Articles
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

Glioblastoma, previously known as glioblastoma multiforme (GBM), is the most aggressive and most common type of cancer that originates in the brain, and has a very poor prognosis for survival. Initial signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness.
An angiogenesis inhibitor is a substance that inhibits the growth of new blood vessels (angiogenesis). Some angiogenesis inhibitors are endogenous and a normal part of the body's control and others are obtained exogenously through pharmaceutical drugs or diet.
Intravasation is the invasion of cancer cells through the basement membrane into a blood or lymphatic vessel. Intravasation is one of several carcinogenic events that initiate the escape of cancerous cells from their primary sites. Other mechanisms include invasion through basement membranes, extravasation, and colonization of distant metastatic sites. Cancer cell chemotaxis also relies on this migratory behavior to arrive at a secondary destination designated for cancer cell colonization.

Fast neutron therapy utilizes high energy neutrons typically between 50 and 70 MeV to treat cancer. Most fast neutron therapy beams are produced by reactors, cyclotrons (d+Be) and linear accelerators. Neutron therapy is currently available in Germany, Russia, South Africa and the United States. In the United States, one treatment center is operational, in Seattle, Washington. The Seattle center uses a cyclotron which produces a proton beam impinging upon a beryllium target.

Tomotherapy is a type of radiation therapy treatment machine. In tomotherapy a thin radiation beam is modulated as it rotates around the patient, while they are moved through the bore of the machine. The name comes from the use of a strip-shaped beam, so that only one “slice” of the target is exposed at any one time by the radiation. The external appearance of the system and movement of the radiation source and patient can be considered analogous to a CT scanner, which uses lower doses of radiation for imaging. Like a conventional machine used for X-ray external beam radiotherapy, a linear accelerator generates the radiation beam, but the external appearance of the machine, the patient positioning, and treatment delivery is different. Conventional linacs do not work on a slice-by-slice basis but typically have a large area beam which can also be resized and modulated.

Steven Kenneth Libutti, M.D., F.A.C.S. is an American surgeon and scientist. In January 2017, he became the third permanent Director of the Rutgers Cancer Institute of New Jersey, Vice Chancellor for Cancer Programs for Rutgers Biomedical and Health Sciences and the Senior Vice President for Oncology Services for RWJBarnabas Health, the largest health system in New Jersey. He is a tenured Distinguished Professor of Surgery at the Rutgers Robert Wood Johnson Medical School. Libutti's work on the study of tumor angiogenesis and the tumor microenvironment has led to novel approaches for the treatment of cancer. He is also one of the pioneers of regional and targeted cancer therapy.

Paclitaxel trevatide is an experimental chemotherapy drug that is under development by Angiochem Inc, a Canadian biotech company. Phase II clinical trials have completed for several indications, and the company is preparing for phase III trials.
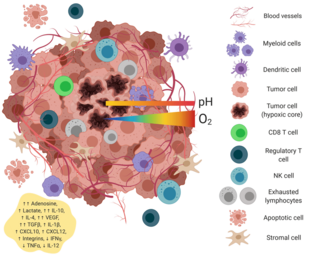
The tumor microenvironment is a complex ecosystem surrounding a tumor, composed of cancer cells, stromal tissue and the extracellular matrix. Mutual interaction between cancer cells and the different components of the tumor microenvironment support its growth and invasion in healthy tissues which correlates with tumor resistance to current treatments and poor prognosis. The tumor microenvironment is in constant change because of the tumor's ability to influence the microenvironment by releasing extracellular signals, promoting tumor angiogenesis and inducing peripheral immune tolerance, while the immune cells in the microenvironment can affect the growth and evolution of cancerous cells.
The stresses, one of the physical hallmarks of cancer, is exerted by the solid components of a tissue and accumulated within solid structural components during growth and progression.
William Mark Saltzman was named the Goizueta Foundation Professor of Biomedical and Chemical Engineering at Yale University on July 1, 2002 and became the founding chair of Yale's Department of Biomedical Engineering in 2003. Saltzman's research aims to promote new methods for drug delivery and develop new biotechnologies to combat human disease. A pioneer in the fields of biomaterials, nanobiotechnology, and tissue engineering, Saltzman has contributed to the design and implementation of a number of clinical technologies that have become essential to medical practice today. His popular course Frontiers of Biomedical Engineering is available to everyone through Open Yale Courses.

Holger Lode is a German specialist for pediatrics. He is Professor and Chair of the Department of General Pediatrics and Pediatric Hematology and Oncology at the University Medicine Greifswald. He is also the director of the Center of Pediatrics and Adolescent Medicine in Greifswald. Lode is well known for his clinical and scientific work on immunotherapy of neuroblastoma.

Tumor-associated endothelial cells or tumor endothelial cells (TECs) refers to cells lining the tumor-associated blood vessels that control the passage of nutrients into surrounding tumor tissue. Across different cancer types, tumor-associated blood vessels have been discovered to differ significantly from normal blood vessels in morphology, gene expression, and functionality in ways that promote cancer progression. There has been notable interest in developing cancer therapeutics that capitalize on these abnormalities of the tumor-associated endothelium to destroy tumors.
The host response to cancer therapy is defined as a physiological response of the non-malignant cells of the body to a specific cancer therapy. The response is therapy-specific, occurring independently of cancer type or stage.
Yun Chae-ok is a Korean-born scientist and professor of Bioengineering at Hanyang University, Seoul, Korea.
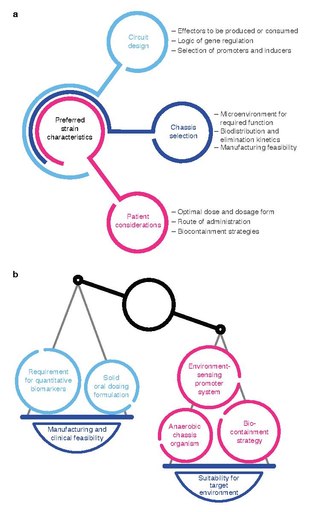
Bacterial therapy is the therapeutic use of bacteria to treat diseases. Bacterial therapeutics are living medicines, and may be wild type bacteria or bacteria that have been genetically engineered to possess therapeutic properties that is injected into a patient. Other examples of living medicines include cellular therapeutics, activators of anti-tumor immunity, or synergizing with existing tools and approaches. and phage therapeutics, or as delivery vehicles for treatment, diagnosis, or imaging, complementing or synergizing with existing tools and approaches.

Debra Auguste is an American chemical engineer and professor at Northeastern University in the department of chemical engineering. Auguste is dedicated to developing treatments for triple negative breast cancer, one of the most aggressive and fatal cancers that disproportionately affects African American women. Her lab characterizes biomarkers of triple negative breast cancer and develops novel biocompatible therapeutic technologies to target and destroy metastatic cancer cells. Auguste received the 2012 Presidential Early Career Award for Scientists and Engineers and in 2010 was named in the 50 Most Influential African-Americans in Technology. In 2020, Auguste became an Elected Fellow of the American Institute for Medical and Biological Engineering.
Priyabrata Mukherjee is an American, academic researcher and professor. He is a Presbyterian Health Foundation presidential professor at the University of Oklahoma Health Sciences Center and associate director for translational research at Stephenson Cancer Center at the OU Health Sciences Center. He also holds the Peggy and Charles Stephenson endowed chair in cancer laboratory research at the OU Health Sciences Center.

Stacey Finley is the Nichole A. and Thuan Q. Pham Professor and associate professor of chemical engineering and materials science, and quantitative and computational biology at the University of Southern California. Finley has a joint appointment in the department of chemical engineering and materials science, and she is a member of the USC Norris Comprehensive Cancer Center. Finley is also a standing member of the MABS Study Section at NIH. Her research has been supported by grants from the NSF, NIH, and American Cancer Society.
Ravi Radhakrishnan is an American engineer and an academic. He is the Herman P. Schwan Chair of Bioengineering as well as a professor in the Department of Chemical and Biomolecular Engineering at the University of Pennsylvania.
References
- ↑ Steele Laboratories web site
- ↑ Jain, RK. Barriers to drug delivery in solid tumors. Sci Am, 271: 58-65, 1994
- ↑ Jain, RK. Taming vessels to treat cancer. Sci Am, 298: 56-63, 2008
- ↑ Jain, RK. An indirect way to tame cancer. Sci Am, 310: 46-53, 2014
- ↑ Jain, RK and PF Carmeliet. Vessels of death or life. Sci Am, 285: 38-45, 2001
- ↑ Jain, RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol, 31: 2205-18, 2013
- ↑ Video of the award lecture, Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers; 2012 ASCO Annual Meeting
- ↑ Jain, RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell, 26: 605-22, 2014
- ↑ Jain, RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med, 7: 987-9, 2001
- ↑ Jain, RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science, 307: 58-62, 2005
- 1 2 ASCO Post, August 15, 2014; Vol. 5, Issue 13
- ↑ Thomson-Reuters list of highly cited researchers
- ↑ National Science & Technology Medals Foundation