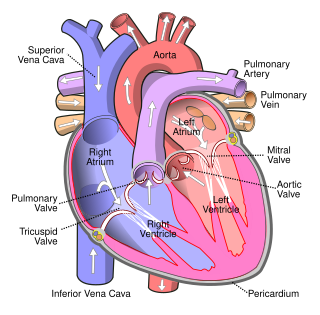
The mitral valve, also known as the bicuspid valve or left atrioventricular valve, is one of the four heart valves. It has two cusps or flaps and lies between the left atrium and the left ventricle of the heart. The heart valves are all one-way valves allowing blood flow in just one direction. The mitral valve and the tricuspid valve are known as the atrioventricular valves because they lie between the atria and the ventricles.

Systole is the part of the cardiac cycle during which some chambers of the heart contract after refilling with blood.

A ventricle is one of two large chambers located toward the bottom of the heart that collect and expel blood towards the peripheral beds within the body and lungs. The blood pumped by a ventricle is supplied by an atrium, an adjacent chamber in the upper heart that is smaller than a ventricle. Interventricular means between the ventricles, while intraventricular means within one ventricle.

Echocardiography, also known as cardiac ultrasound, is the use of ultrasound to examine the heart. It is a type of medical imaging, using standard ultrasound or Doppler ultrasound. The visual image formed using this technique is called an echocardiogram, a cardiac echo, or simply an echo.
In cardiovascular physiology, stroke volume (SV) is the volume of blood pumped from the left ventricle per beat. Stroke volume is calculated using measurements of ventricle volumes from an echocardiogram and subtracting the volume of the blood in the ventricle at the end of a beat from the volume of blood just prior to the beat. The term stroke volume can apply to each of the two ventricles of the heart, although it usually refers to the left ventricle. The stroke volumes for each ventricle are generally equal, both being approximately 70 mL in a healthy 70-kg man.
An ejection fraction (EF) is the volumetric fraction of fluid ejected from a chamber with each contraction. It can refer to the cardiac atrium, ventricle, gall bladder, or leg veins, although if unspecified it usually refers to the left ventricle of the heart. EF is widely used as a measure of the pumping efficiency of the heart and is used to classify heart failure types. It is also used as an indicator of the severity of heart failure, although it has recognized limitations.
End-systolic volume (ESV) is the volume of blood in a ventricle at the end of contraction, or systole, and the beginning of filling, or diastole.

Afterload is the pressure that the heart must work against to eject blood during systole. Afterload is proportional to the average arterial pressure. As aortic and pulmonary pressures increase, the afterload increases on the left and right ventricles respectively. Afterload changes to adapt to the continually changing demands on an animal's cardiovascular system. Afterload is proportional to mean systolic blood pressure and is measured in millimeters of mercury.

Diastole is the relaxed phase of the cardiac cycle when the chambers of the heart are refilling with blood. The contrasting phase is systole when the heart chambers are contracting. Atrial diastole is the relaxing of the atria, and ventricular diastole the relaxing of the ventricles.

In cardiac physiology, preload is the amount of sarcomere stretch experienced by cardiac muscle cells, called cardiomyocytes, at the end of ventricular filling during diastole. Preload is directly related to ventricular filling. As the relaxed ventricle fills during diastole, the walls are stretched and the length of sarcomeres increases. Sarcomere length can be approximated by the volume of the ventricle because each shape has a conserved surface-area-to-volume ratio. This is useful clinically because measuring the sarcomere length is destructive to heart tissue. It requires cutting out a piece of cardiac muscle to look at the sarcomeres under a microscope. It is currently not possible to directly measure preload in the beating heart of a living animal. Preload is estimated from end-diastolic ventricular pressure and is measured in millimeters of mercury (mmHg).

The cardiac cycle is the performance of the human heart from the beginning of one heartbeat to the beginning of the next. It consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, called systole. After emptying, the heart relaxes and expands to receive another influx of blood returning from the lungs and other systems of the body, before again contracting to pump blood to the lungs and those systems. A normally performing heart must be fully expanded before it can efficiently pump again. Assuming a healthy heart and a typical rate of 70 to 75 beats per minute, each cardiac cycle, or heartbeat, takes about 0.8 second to complete the cycle.
The apex beat, also called the apical impulse, is the pulse felt at the point of maximum impulse (PMI), which is the point on the precordium farthest outwards (laterally) and downwards (inferiorly) from the sternum at which the cardiac impulse can be felt. The cardiac impulse is the vibration resulting from the heart rotating, moving forward, and striking against the chest wall during systole. The PMI is not the apex of the heart but is on the precordium not far from it. Another theory for the occurrence of the PMI is the early systolic contraction of the longitudinal fibers of the left ventricle located on the endocardial surface of this chamber. This period of the cardiac cycle is called isovolumic contraction. Because the contraction starts near the base of the left ventricle and spreads toward the apex most of the longitudinal fibers of the left ventricle have shortened before the apex. The rapidly increasing pressure developed by the shortening of these fibers causes the aortic valve to open and the apex to move outward causing the PMI. Anatomical dissection of the musculature of the apex reveals that muscle fibers are no longer longitudinal oriented but form a spiral mass of muscular tissues which may also have an effect on the ability of the apex to contract longitudinally. After the longitudinal fibers contract, the ejection of blood out of the left ventricle is accomplished by the torsional action of the circumferential muscle fibers of the left ventricle that are in the mid-portion of the ventricle and contract after the longitudinal fibers. During the longitudinal fiber contraction, the volume of the left ventricle has not changed keeping the apex in intimate contact with the chest wall allowing the ability to feel the apex move outward before the heart empties greater than 55% of its volume and the apex falling away from the chest wall.
The E/A ratio is a marker of the function of the left ventricle of the heart. It represents the ratio of peak velocity blood flow from left ventricular relaxation in early diastole to peak velocity flow in late diastole caused by atrial contraction. It is calculated using Doppler echocardiography, an ultrasound-based cardiac imaging modality. Abnormalities in the E/A ratio suggest that the left ventricle, which pumps blood into the systemic circulation, cannot fill with blood properly in the period between contractions. This phenomenon is referred to as diastolic dysfunction and can eventually lead to the symptoms of heart failure.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.
A plot of a system's pressure versus volume has long been used to measure the work done by the system and its efficiency. This analysis can be applied to heat engines and pumps, including the heart. A considerable amount of information on cardiac performance can be determined from the pressure vs. volume plot. A number of methods have been determined for measuring PV-loop values experimentally.

In the fields of cardiology and medical imaging, speckle tracking echocardiography (STE) is an echocardiographic imaging technique. It analyzes the motion of tissues in the heart by using the naturally occurring speckle pattern in the myocardium.
Tissue Doppler echocardiography (TDE) is a medical ultrasound technology, specifically a form of echocardiography that measures the velocity of the heart muscle (myocardium) through the phases of one or more heartbeats by the Doppler effect of the reflected ultrasound. The technique is the same as for flow Doppler echocardiography measuring flow velocities. Tissue signals, however, have higher amplitude and lower velocities, and the signals are extracted by using different filter and gain settings. The terms tissue Doppler imaging (TDI) and tissue velocity imaging (TVI) are usually synonymous with TDE because echocardiography is the main use of tissue Doppler.

Strain rate imaging is a method in echocardiography for measuring regional or global deformation of the myocardium. The term "deformation" refers to the myocardium changing shape and dimensions during the cardiac cycle. If there is myocardial ischemia, or there has been a myocardial infarction, in part of the heart muscle, this part is weakened and shows reduced and altered systolic function. Also in regional asynchrony, as in bundle branch block, there is regional heterogeneity of systolic function. By strain rate imaging, the simultaneous function of different regions can be displayed and measured. The method was first based on colour tissue Doppler. by using the longitudinal myocardial velocity gradient, already in use transmurally. Later, the regional deformation has also been available by speckle tracking echocardiography, both methods having some, but different methodological weaknesses. Both methods, however, will acquire the same data, and also can be displayed by the same type of display.
In clinical cardiology the term "diastolic function" is most commonly referred as how the heart fills. Parallel to "diastolic function", the term "systolic function" is usually referenced in terms of the left ventricular ejection fraction (LVEF), which is the ratio of stroke volume and end-diastolic volume. Due to the epidemic of heart failure, particularly the cases determined as diastolic heart failure, it is increasingly urgent and crucial to understand the meaning of “diastolic function”. Unlike "systolic function", which can be simply evaluated by LVEF, there are no established dimensionless parameters for "diastolic function" assessment. Hence to further study "diastolic function" the complicated and speculative physiology must be taken into consideration.
The main pathophysiology of heart failure is a reduction in the efficiency of the heart muscle, through damage or overloading. As such, it can be caused by a wide number of conditions, including myocardial infarction, hypertension and cardiac amyloidosis. Over time these increases in workload will produce changes to the heart itself:







