
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution, and may require a mordant to improve the fastness of the dye on the fiber.

A toxic heavy metal is any relatively dense metal or metalloid that is noted for its potential toxicity, especially in environmental contexts. The term has particular application to cadmium, mercury and lead, all of which appear in the World Health Organization's list of 10 chemicals of major public concern. Other examples include manganese, chromium, cobalt, nickel, copper, zinc, silver, antimony and thallium.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. Adsorption is a surface phenomenon, while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term sorption encompasses both processes, while desorption is the reverse of it.

Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use. This treatment is crucial to human health and allows humans to benefit from both drinking and irrigation use.
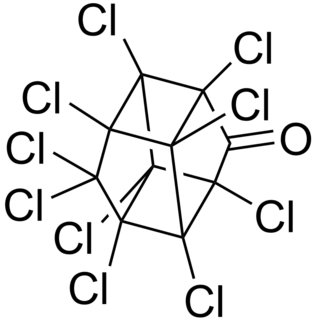
Chlordecone, better known in the United States under the brand name Kepone, is an organochlorine compound and a colourless solid. It is an obsolete insecticide, related to Mirex and DDT. Its use was so disastrous that it is now prohibited in the western world, but only after many thousands of tonnes had been produced and used. Chlordecone is a known persistent organic pollutant (POP) that was banned globally by the Stockholm Convention on Persistent Organic Pollutants in 2009.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area available for adsorption or chemical reactions. Activation is analogous to making popcorn from dried corn kernels: popcorn is light, fluffy, and has a surface area that is much larger than the kernels. Activated is sometimes replaced by active.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

A bioreactor refers to any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel. It may also refer to a device or system designed to grow cells or tissues in the context of cell culture. These devices are being developed for use in tissue engineering or biochemical/bioprocess engineering.
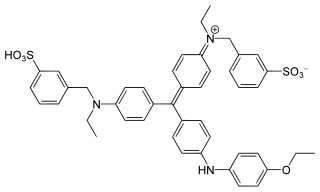
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries.

Brilliant blue FCF is a synthetic organic compound used primarily as a blue colorant for processed foods, medications, dietary supplements, and cosmetics. It is classified as a triarylmethane dye and is known under various names, such as FD&C Blue No. 1 or acid blue 9. It is denoted by E number E133 and has a color index of 42090. It has the appearance of a blue powder and is soluble in water and glycerol, with a maximum absorption at about 628 nanometers. It is one of the oldest FDA-approved color additives and is generally considered nontoxic and safe.

Mycoremediation is a form of bioremediation in which fungi-based remediation methods are used to decontaminate the environment. Fungi have been proven to be a cheap, effective and environmentally sound way for removing a wide array of contaminants from damaged environments or wastewater. These contaminants include heavy metals, organic pollutants, textile dyes, leather tanning chemicals and wastewater, petroleum fuels, polycyclic aromatic hydrocarbons, pharmaceuticals and personal care products, pesticides and herbicides in land, fresh water, and marine environments.
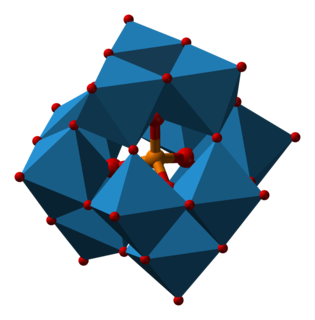
Phosphotungstic acid (PTA) or tungstophosphoric acid (TPA), is a heteropoly acid with the chemical formula H3PW12O40]. It forms hydrates H3[PW12O40]·nH2O. It is normally isolated as the n = 24 hydrate but can be desiccated to the hexahydrate. EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly yellow-green crystals, with melting point 89 °C. It is odorless and soluble in water. It is not especially toxic, but is a mild acidic irritant. The compound is known by a variety of names and acronyms.

Arsenic contamination of groundwater is a form of groundwater pollution which is often due to naturally occurring high concentrations of arsenic in deeper levels of groundwater. It is a high-profile problem due to the use of deep tube wells for water supply in the Ganges Delta, causing serious arsenic poisoning to large numbers of people. A 2007 study found that over 137 million people in more than 70 countries are probably affected by arsenic poisoning of drinking water. The problem became serious health concern after mass poisoning of water in Bangladesh. Arsenic contamination of ground water is found in many countries throughout the world, including the US.

For the parent molecule 9,10-anthraquinone, see anthraquinone

Ischnoderma is a genus of polypore fungi. Species in the genus have dark brown and tomentose fruit bodies that become darker brown to black and smooth when mature. The genus, widespread in temperate regions, contains an estimated 10 species.

Azolla pinnata is a species of fern known by several common names, including mosquitofern, feathered mosquitofern and water velvet. It is native to much of Africa, Asia and parts of Australia. It is an aquatic plant, it is found floating upon the surface of the water. It grows in quiet and slow-moving water bodies, because swift currents and waves break up the plant. At maximum growth rate, it can double its biomass in 1.9 days, with most strains attaining such growth within a week under optimal conditions.

Acid Blue 25 (C20H13N2NaO5S) is an acid dye that is water-soluble and anionic and used for adsorption research. The structure is an anthraquinone.

Industrial dye degradation is any of a number of processed by which dyes are broken down, ideally into innocuous products. Many dyes, specifically in the textile industry such as methylene blue or methyl red, are released into ecosystems through water waste. Many of these dyes can be carcinogenic. In paper recycling dyes can be removed from fibres during a deinking stage prior to degradation.
Dye-ligand affinity chromatography is one of the Affinity chromatography techniques used for protein purification of a complex mixture. Like general chromatography, but using dyes to apply on a support matrix of a column as the stationary phase that will allow a range of proteins with similar active sites to bind to, refers to as pseudo-affinity. Synthetic dyes are used to mimic substrates or cofactors binding to the active sites of proteins which can be further enhanced to target more specific proteins. Follow with washing, the process of removing other non-target molecules, then eluting out target proteins out by changing pH or manipulate the salt concentration. The column can be reused many times due to the stability of immobilized dyes. It can carry out in a conventional way by using as a packed column, or in high-performance liquid chromatography (HPLC) column.


















