Related Research Articles
The consciousness and binding problem is the problem of how objects, background and abstract or emotional features are combined into a single experience.
A gamma wave or gamma rhythm is a pattern of neural oscillation in humans with a frequency between 25 and 140 Hz, the 40 Hz point being of particular interest. Gamma rhythms are correlated with large-scale brain network activity and cognitive phenomena such as working memory, attention, and perceptual grouping, and can be increased in amplitude via meditation or neurostimulation. Altered gamma activity has been observed in many mood and cognitive disorders such as Alzheimer's disease, epilepsy, and schizophrenia. Elevated gamma activity has also been observed in moments preceding death.
The N400 is a component of time-locked EEG signals known as event-related potentials (ERP). It is a negative-going deflection that peaks around 400 milliseconds post-stimulus onset, although it can extend from 250-500 ms, and is typically maximal over centro-parietal electrode sites. The N400 is part of the normal brain response to words and other meaningful stimuli, including visual and auditory words, sign language signs, pictures, faces, environmental sounds, and smells.

Neural binding is the neuroscientific aspect of what is commonly known as the binding problem: the interdisciplinary difficulty of creating a comprehensive and verifiable model for the unity of consciousness. "Binding" refers to the integration of highly diverse neural information in the forming of one's cohesive experience. The neural binding hypothesis states that neural signals are paired through synchronized oscillations of neuronal activity that combine and recombine to allow for a wide variety of responses to context-dependent stimuli. These dynamic neural networks are thought to account for the flexibility and nuanced response of the brain to various situations. The coupling of these networks is transient, on the order of milliseconds, and allows for rapid activity.
Sensitization is a non-associative learning process in which repeated administration of a stimulus results in the progressive amplification of a response. Sensitization often is characterized by an enhancement of response to a whole class of stimuli in addition to the one that is repeated. For example, repetition of a painful stimulus may make one more responsive to a loud noise.

In cognitive science and neuropsychology, executive functions are a set of cognitive processes that are necessary for the cognitive control of behavior: selecting and successfully monitoring behaviors that facilitate the attainment of chosen goals. Executive functions include basic cognitive processes such as attentional control, cognitive inhibition, inhibitory control, working memory, and cognitive flexibility. Higher-order executive functions require the simultaneous use of multiple basic executive functions and include planning and fluid intelligence.
Sensory neuroscience is a subfield of neuroscience which explores the anatomy and physiology of neurons that are part of sensory systems such as vision, hearing, and olfaction. Neurons in sensory regions of the brain respond to stimuli by firing one or more nerve impulses following stimulus presentation. How is information about the outside world encoded by the rate, timing, and pattern of action potentials? This so-called neural code is currently poorly understood and sensory neuroscience plays an important role in the attempt to decipher it. Looking at early sensory processing is advantageous since brain regions that are "higher up" contain neurons which encode more abstract representations. However, the hope is that there are unifying principles which govern how the brain encodes and processes information. Studying sensory systems is an important stepping stone in our understanding of brain function in general.
Neural coding is a neuroscience field concerned with characterising the hypothetical relationship between the stimulus and the individual or ensemble neuronal responses and the relationship among the electrical activity of the neurons in the ensemble. Based on the theory that sensory and other information is represented in the brain by networks of neurons, it is thought that neurons can encode both digital and analog information.
The mismatch negativity (MMN) or mismatch field (MMF) is a component of the event-related potential (ERP) to an odd stimulus in a sequence of stimuli. It arises from electrical activity in the brain and is studied within the field of cognitive neuroscience and psychology. It can occur in any sensory system, but has most frequently been studied for hearing and for vision, in which case it is abbreviated to vMMN. The (v)MMN occurs after an infrequent change in a repetitive sequence of stimuli For example, a rare deviant (d) stimulus can be interspersed among a series of frequent standard (s) stimuli. In hearing, a deviant sound can differ from the standards in one or more perceptual features such as pitch, duration, loudness, or location. The MMN can be elicited regardless of whether someone is paying attention to the sequence. During auditory sequences, a person can be reading or watching a silent subtitled movie, yet still show a clear MMN. In the case of visual stimuli, the MMN occurs after an infrequent change in a repetitive sequence of images.

Negative priming is an implicit memory effect in which prior exposure to a stimulus unfavorably influences the response to the same stimulus. It falls under the category of priming, which refers to the change in the response towards a stimulus due to a subconscious memory effect. Negative priming describes the slow and error-prone reaction to a stimulus that is previously ignored. For example, a subject may be imagined trying to pick a red pen from a pen holder. The red pen becomes the target of attention, so the subject responds by moving their hand towards it. At this time, they mentally block out all other pens as distractors to aid in closing in on just the red pen. After repeatedly picking the red pen over the others, switching to the blue pen results in a momentary delay picking the pen out. The slow reaction due to the change of the distractor stimulus to target stimulus is called the negative priming effect.
In cognitive neuroscience, visual modularity is an organizational concept concerning how vision works. The way in which the primate visual system operates is currently under intense scientific scrutiny. One dominant thesis is that different properties of the visual world require different computational solutions which are implemented in anatomically/functionally distinct regions that operate independently – that is, in a modular fashion.
Priming is the idea that exposure to one stimulus may influence a response to a subsequent stimulus, without conscious guidance or intention. The priming effect refers to the positive or negative effect of a rapidly presented stimulus on the processing of a second stimulus that appears shortly after. Generally speaking, the generation of priming effect depends on the existence of some positive or negative relationship between priming and target stimuli. For example, the word nurse might be recognized more quickly following the word doctor than following the word bread. Priming can be perceptual, associative, repetitive, positive, negative, affective, semantic, or conceptual. Priming effects involve word recognition, semantic processing, attention, unconscious processing, and many other issues, and are related to differences in various writing systems. Research, however, has yet to firmly establish the duration of priming effects, yet their onset can be almost instantaneous.
The oddball paradigm is an experimental design used within psychology research. Presentations of sequences of repetitive stimuli are infrequently interrupted by a deviant stimulus. The reaction of the participant to this "oddball" stimulus is recorded.
Chronostasis is a type of temporal illusion in which the first impression following the introduction of a new event or task-demand to the brain can appear to be extended in time. For example, chronostasis temporarily occurs when fixating on a target stimulus, immediately following a saccade. This elicits an overestimation in the temporal duration for which that target stimulus was perceived. This effect can extend apparent durations by up to half a second and is consistent with the idea that the visual system models events prior to perception.
Many experiments have been done to find out how the brain interprets stimuli and how animals develop fear responses. The emotion, fear, has been hard-wired into almost every individual, due to its vital role in the survival of the individual. Researchers have found that fear is established unconsciously and that the amygdala is involved with fear conditioning.
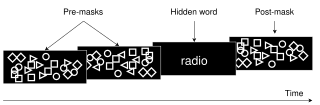
Visual masking is a phenomenon of visual perception. It occurs when the visibility of one image, called a target, is reduced by the presence of another image, called a mask. The target might be invisible or appear to have reduced contrast or lightness. There are three different timing arrangements for masking: forward masking, backward masking, and simultaneous masking. In forward masking, the mask precedes the target. In backward masking the mask follows the target. In simultaneous masking, the mask and target are shown together. There are two different spatial arrangements for masking: pattern masking and metacontrast. Pattern masking occurs when the target and mask locations overlap. Metacontrast masking occurs when the mask does not overlap with the target location.
Surround suppression is where the relative firing rate of a neuron may under certain conditions decrease when a particular stimulus is enlarged. It has been observed in electrophysiology studies of the brain and has been noted in many sensory neurons, most notably in the early visual system. Surround suppression is defined as a reduction in the activity of a neuron in response to a stimulus outside its classical receptive field.
The bi-directional hypothesis of language and action proposes that the sensorimotor and language comprehension areas of the brain exert reciprocal influence over one another. This hypothesis argues that areas of the brain involved in movement and sensation, as well as movement itself, influence cognitive processes such as language comprehension. In addition, the reverse effect is argued, where it is proposed that language comprehension influences movement and sensation. Proponents of the bi-directional hypothesis of language and action conduct and interpret linguistic, cognitive, and movement studies within the framework of embodied cognition and embodied language processing. Embodied language developed from embodied cognition, and proposes that sensorimotor systems are not only involved in the comprehension of language, but that they are necessary for understanding the semantic meaning of words.
Social cognitive neuroscience is the scientific study of the biological processes underpinning social cognition. Specifically, it uses the tools of neuroscience to study "the mental mechanisms that create, frame, regulate, and respond to our experience of the social world". Social cognitive neuroscience uses the epistemological foundations of cognitive neuroscience, and is closely related to social neuroscience. Social cognitive neuroscience employs human neuroimaging, typically using functional magnetic resonance imaging (fMRI). Human brain stimulation techniques such as transcranial magnetic stimulation and transcranial direct-current stimulation are also used. In nonhuman animals, direct electrophysiological recordings and electrical stimulation of single cells and neuronal populations are utilized for investigating lower-level social cognitive processes.

Laura Busse is a German neuroscientist and professor of Systemic Neuroscience within the Division of Neurobiology at the Ludwig Maximilian University of Munich. Busse's lab studies context-dependent visual processing in mouse models by performing large scale in vivo electrophysiological recordings in the thalamic and cortical circuits of awake and behaving mice.
References
- ↑ Schacter, D.L; Buckner, R.L (1998). "Priming and the brain". Neuron. 20 (2): 185–195. doi: 10.1016/s0896-6273(00)80448-1 . PMID 9491981. S2CID 5872964.
- ↑ Maljkovic, V; Nakayama, K (1994). "Priming of pop-out: I. Role of features". Memory & Cognition. 22 (6): 657–672. doi: 10.3758/bf03209251 . PMID 7808275.
- ↑ Biederman, I; Cooper, E.E (1991). "Evidence for complete translational and reflectional invariance in visual object recognition". Perception. 20 (5): 585–593. doi:10.1068/p200585. PMID 1806902. S2CID 21635280.
- ↑ Biederman, I; Cooper, E.E (1992). "Size invariance in visual object recognition". Journal of Experimental Psychology: Human Perception and Performance. 18 (1): 121–133. doi:10.1037/0096-1523.18.1.121.
- ↑ Fiser, J; Biederman, I (2001). "Invariance of long-term visual priming to scale, reflection, translation, and hemisphere". Vision Research. 41 (2): 221–234. doi: 10.1016/s0042-6989(00)00234-0 . PMID 11163856. S2CID 17656028.
- ↑ Huber, D.E; Clark, T.F; Curran, T; Winkielman, P (2008). "Effects of repetition priming on recognition memory: Testing a perceptual fluency-disfluency model". Journal of Experimental Psychology: Learning, Memory, and Cognition. 34 (6): 1305–1324. doi:10.1037/a0013370. PMID 18980396.
- ↑ Henson, R.N; Rylands, A; Ross, E; Vuilleumeir, P; Rugg, M.D (2004). "The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming". NeuroImage. 21 (4): 1674–1689. doi:10.1016/j.neuroimage.2003.12.020. PMID 15050590. S2CID 10102927.
- ↑ Rugg, M.D; Mark, R.E; Walla, P; Schloerscheidt, A.M; Birch, C.S; Allan, K (1998). "Dissociation of the neural correlates of implicit and explicit memory". Nature. 392 (6676): 595–598. Bibcode:1998Natur.392..595R. doi:10.1038/33396. PMID 9560154. S2CID 2390465.
- ↑ Kinder, A; Shanks, D.R (2001). "Amnesia and the declarative/nondeclarative distinction: A recurrent network model of classification, recognition, and repetition priming". Journal of Cognitive Neuroscience. 13 (5): 648–669. doi:10.1162/089892901750363217. PMID 11506662. S2CID 35579825.
- ↑ Fleischman, D.A (2007). "Repetition priming in aging and Alzheimer's disease: an integrative review and future directions". Cortex. 43 (7): 889–897. doi:10.1016/s0010-9452(08)70688-9. PMID 17941347. S2CID 4476574.
- ↑ Reder, L.M; Park, H; Kieffaber, P.D (2009). "Memory systems do not divide on consciousness: Reinterpreting memory in terms of activation and binding". Psychological Bulletin. 135 (1): 23–49. doi:10.1037/a0013974. PMC 2747326 . PMID 19210052.
- 1 2 Larsson, J; Smith, A.T (2012). "fMRI repetition suppression: neuronal adaptation or stimulus expectation?". Cerebral Cortex. 22 (3): 567–576. doi:10.1093/cercor/bhr119. PMC 3278317 . PMID 21690262.
- 1 2 3 Summerfield, C; Egner, T; Greene, M; Koechlin, E; Mangels, J; Hirsch, J (2006). "Predictive codes for forthcoming perception in the frontal cortex". Science. 314 (5803): 1311–1314. Bibcode:2006Sci...314.1311S. doi:10.1126/science.1132028. PMID 17124325. S2CID 13986747.
- ↑ Neill, W.T (1997). "Episodic retrieval in negative priming and repetition priming". Journal of Experimental Psychology: Learning, Memory, and Cognition. 23 (6): 1291–3105. doi:10.1037/0278-7393.23.6.1291.
- 1 2 Grill-Spector, K; Malach, R (2001). "fMR-adaptation: a tool for studying the functional properties of human cortical neurons". Acta Psychologica. 107 (1): 293–321. doi:10.1016/s0001-6918(01)00019-1. PMID 11388140.
- ↑ Sobotka, S; Ringo, J.L (1994). "Stimulus specific adaptation in excited but not in inhibited cells in inferotemporal cortex of macaque". Brain Research. 646 (1): 95–99. doi:10.1016/0006-8993(94)90061-2. PMID 8055344. S2CID 31639947.
- ↑ Buckner, R.L; Koustaal, W (1998). "Functional neuroimaging studies of encoding, priming, and explicit memory retrieval". Proc. Natl. Acad. Sci. U.S.A. 95 (3): 891–898. Bibcode:1998PNAS...95..891B. doi: 10.1073/pnas.95.3.891 . PMC 33813 . PMID 9448256.
- ↑ Schendan, H.E; Kutas, M (2003). "Time course of processes and representations supporting visual object identification and memory". Journal of Cognitive Neuroscience. 15 (1): 111–135. doi:10.1162/089892903321107864. PMID 12590847. S2CID 15024510.
- ↑ Gilbert, J (2005). "Top-down modulation during object priming: evidence from MEG". Journal of Cognitive Neuroscience. Supplement: 132.
- ↑ Pobric, G; Schweinberger, S.R; Lavidor, M (2007). "Magnetic stimulation of the right visual cortex impairs form-specific priming" (PDF). Journal of Cognitive Neuroscience. 19 (6): 1013–1020. doi:10.1162/jocn.2007.19.6.1013. PMID 17536971. S2CID 2236273.
- 1 2 Gagnepain, P; Chételat, G; Landeau, B; Dayan, J; Eustache, F; Lebreton, K (2008). "Spoken word memory traces within the human auditory cortex revealed by repetition priming and functional magnetic resonance imaging". The Journal of Neuroscience. 28 (20): 5281–5289. doi: 10.1523/jneurosci.0565-08.2008 . PMC 6670649 . PMID 18480284.
- ↑ Olsson, M.J (1999). "Implicit testing of odor memory: instances of positive and negative repetition priming". Chemical Senses. 24 (3): 347–350. doi: 10.1093/chemse/24.3.347 . PMID 10400453.
- ↑ Kohn, A; Movshon, J.A (2003). "Neuronal adaptation to visual motion in area MT of the macaque". Neuron. 39 (4): 681–691. doi: 10.1016/s0896-6273(03)00438-0 . PMID 12925281. S2CID 1826117.
- 1 2 3 Grill-Spector, K; Henson, R; Martin, A (2006). "Repetition and the brain: neural models of stimulus-specific effects". Trends in Cognitive Sciences. 10 (1): 14–23. doi:10.1016/j.tics.2005.11.006. PMID 16321563. S2CID 8996826.
- ↑ Dragoi, V; Sharma, J; Miller, E.K; Sur, M (2002). "Dynamics of neuronal sensitivity in visual cortex and local feature discrimination". Nature Neuroscience. 5 (9): 883–891. doi:10.1038/nn900. PMID 12161755. S2CID 13526431.
- ↑ Wiggs, C.L; Martin, A (1998). "Properties and mechanisms of perceptual priming". Current Opinion in Neurobiology. 8 (2): 227–233. doi:10.1016/s0959-4388(98)80144-x. PMID 9635206. S2CID 9770978.
- ↑ Freedman, D.J; Riesenhuber, M; Poggio, T; Miller, E.K (2006). "Experience-dependent sharpening of visual shape selectivity in inferior temporal cortex". Cerebral Cortex. 16 (11): 1631–1644. doi: 10.1093/cercor/bhj100 . PMID 16400159.
- ↑ Sigala, N; Logothetis, N.K (2002). "Visual categorization shapes feature selectivity in the primate temporal cortex". Nature. 415 (6869): 318–320. Bibcode:2002Natur.415..318S. doi:10.1038/415318a. hdl: 11858/00-001M-0000-0013-E06C-6 . PMID 11797008. S2CID 4300291.
- ↑ Li, L; Miller, E.K; Desimone, R (1993). "The representation of stimulus familiarity in anterior inferior temporal cortex". Journal of Neurophysiology. 69 (6): 1918–1929. doi:10.1152/jn.1993.69.6.1918. PMID 8350131.
- 1 2 James, T.W; Gauthier, I (2006). "Repetition‐induced changes in BOLD response reflect accumulation of neural activity". Human Brain Mapping. 27 (1): 37–46. doi:10.1002/hbm.20165. PMC 6871272 . PMID 15954142.
- ↑ Pedreira, C; Mormann, F; Kraskov, A; Cerf, M; Fried, I; Koch, C; Quiroga, R.Q (2010). "Responses of human medial temporal lobe neurons are modulated by stimulus repetition". Journal of Neurophysiology. 103 (1): 97–107. doi:10.1152/jn.91323.2008. PMC 2807242 . PMID 19864436.
- ↑ Becker, S; Moscovitch, M; Behrmann, M; Joordens, S (1997). "Long-term semantic priming: a computational account and empirical evidence". Journal of Experimental Psychology: Learning, Memory, and Cognition. 23 (5): 1059–1082. doi:10.1037/0278-7393.23.5.1059. PMID 9293622.
- ↑ Friston, K (2005). "A theory of cortical responses". Philosophical Transactions of the Royal Society B: Biological Sciences. 360 (1456): 815–836. doi:10.1098/rstb.2005.1622. PMC 1569488 . PMID 15937014.
- ↑ Rao, R.P; Ballard, D.H (1999). "Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects". Nature Neuroscience. 2 (1): 79–87. doi:10.1038/4580. PMID 10195184.
- ↑ Gilbert, J.R; Gotts, S.J; Carver, F.W; Martin, A (2010). "Object repetition leads to local increases in the temporal coordination of neural responses". Frontiers in Human Neuroscience. 4: 30. doi: 10.3389/fnhum.2010.00030 . PMC 2868300 . PMID 20463867.
- ↑ von Stein, A; Chiang, C; Konig, P (2000). "Top-down processing mediated by interareal synchronization". Proceedings of the National Academy of Sciences of the United States of America. 97 (26): 14748–14753. Bibcode:2000PNAS...9714748V. doi: 10.1073/pnas.97.26.14748 . PMC 18990 . PMID 11121074.
- ↑ Stopfer, M; Laurent, G (1000). "Short-term memory in olfactory network dynamics". Nature. 402 (6762): 664–668. doi:10.1038/45244. PMID 10604472. S2CID 4366918.
- 1 2 3 Ghuman, Avniel S.; Bar, Moshe; Dobbins, Ian G.; Schnyer, David M. (2008-06-17). "The effects of priming on frontal-temporal communication". Proceedings of the National Academy of Sciences of the United States of America. 105 (24): 8405–8409. Bibcode:2008PNAS..105.8405G. doi: 10.1073/pnas.0710674105 . ISSN 1091-6490. PMC 2448849 . PMID 18541919.
- ↑ Gotts, S.J; Chow, C.C; Martin, A (2012). "Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization". Cognitive Neuroscience. 3 (4): 227–237. doi:10.1080/17588928.2012.670617. PMC 3491809 . PMID 23144664.
- ↑ Dobbins, I.G (2004). "Cortical activity reductions during repetition priming can result from rapid response learning". Nature. 428 (6980): 316–319. Bibcode:2004Natur.428..316D. doi:10.1038/nature02400. PMID 14990968. S2CID 3645726.
- ↑ Logan, G.D (1990). "Repetition priming and automaticity: Common underlying mechanisms?". Cognitive Psychology. 22 (1): 1–35. doi:10.1016/0010-0285(90)90002-l. S2CID 9851428.
- ↑ Kunde, W (2003). "Conscious control over the content of unconscious cognition". Cognition. 88 (2): 223–242. doi:10.1016/s0010-0277(03)00023-4. PMID 12763320. S2CID 7038166.
- ↑ Henson, R.N; Eckstein, D; Waszak, F; Frings, C; Horner, A.J (2014). "Stimulus–response bindings in priming". Trends in Cognitive Sciences. 18 (7): 376–384. doi:10.1016/j.tics.2014.03.004. PMC 4074350 . PMID 24768034.
- ↑ Epstein, R.A; Parker, W.E; Feiler, A.M (2008). "Two kinds of fMRI repetition suppression? Evidence for dissociable neural mechanisms". Journal of Neurophysiology. 99 (6): 2877–2886. doi:10.1152/jn.90376.2008. PMID 18400954. S2CID 2907968.
- ↑ Kristjánsson, Á; Campana, G (2010). "Where perception meets memory: A review of repetition priming in visual search tasks". Attention, Perception, & Psychophysics. 72 (1): 5–18. doi:10.3758/app.72.1.5. PMID 20045875. S2CID 46146325.
- ↑ McMahon, D.B; Olson, C.R (2007). "Repetition suppression in monkey inferotemporal cortex: relation to behavioral priming". Journal of Neurophysiology. 97 (5): 3532–3543. doi:10.1152/jn.01042.2006. PMID 17344370.
- ↑ Salimpoor, V.N; Chang, C; Menon, V (2010). "Neural basis of repetition priming during mathematical cognition: repetition suppression or repetition enhancement?". Journal of Cognitive Neuroscience. 22 (4): 790–805. doi:10.1162/jocn.2009.21234. PMC 4366146 . PMID 19366289.
- ↑ Horner, A.J; Henson, R.N (2008). "Priming, response learning and repetition suppression". Neuropsychologia. 46 (7): 1979–1991. doi:10.1016/j.neuropsychologia.2008.01.018. PMC 2430995 . PMID 18328508.
- ↑ Henson, R.N; Shallice, T; Dolan, R (2000). "Neuroimaging evidence for dissociable forms of repetition priming". Science. 287 (5456): 1269–1272. Bibcode:2000Sci...287.1269H. doi:10.1126/science.287.5456.1269. hdl: 21.11116/0000-0001-A15E-0 . PMID 10678834.