Related Research Articles
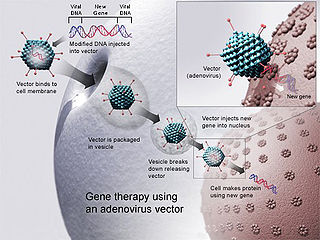
Gene therapy is a medical field which focuses on the utilization of the therapeutic delivery of nucleic acids into a patient's cells as a drug to treat disease. The first attempt at modifying human DNA was performed in 1980 by Martin Cline, but the first successful nuclear gene transfer in humans, approved by the National Institutes of Health, was performed in May 1989. The first therapeutic use of gene transfer as well as the first direct insertion of human DNA into the nuclear genome was performed by French Anderson in a trial starting in September 1990. It is thought to be able to cure many genetic disorders or treat them over time.
Erythropoietin, also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidney in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bone marrow. Low levels of EPO are constantly secreted sufficient to compensate for normal red blood cell turnover. Common causes of cellular hypoxia resulting in elevated levels of EPO include any anemia, and hypoxemia due to chronic lung disease.
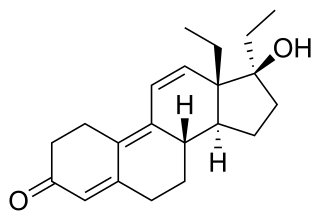
Tetrahydrogestrinone (THG), known by the nickname The Clear, is a synthetic and orally active anabolic–androgenic steroid (AAS) which was never marketed for medical use. It was developed by Patrick Arnold and was used by a number of high-profile athletes such as Marion Jones and Dwain Chambers.

The United States Anti-Doping Agency is a non-profit, non-governmental 501(c)(3) organization and the national anti-doping organization (NADO) for the United States. To protect clean competition and the integrity of sport, USADA provides education, leads scientific initiatives, conducts testing, and oversees the results management process. Headquartered in Colorado Springs, Colorado, USADA is a signatory to the World Anti-Doping Code, which harmonizes anti-doping practices around the world and is widely considered the basis for the strongest and strictest anti-doping programs in sports.
In competitive sports, doping is the use of banned athletic performance-enhancing drugs by athletic competitors. The term doping is widely used by organizations that regulate sporting competitions. The use of drugs to enhance performance is considered unethical, and therefore prohibited, by most international sports organizations, including the International Olympic Committee. Furthermore, athletes taking explicit measures to evade detection exacerbate the ethical violation with overt deception and cheating.
The World Anti-Doping Agency is a foundation initiated by the International Olympic Committee based in Canada to promote, coordinate and monitor the fight against drugs in sports. The agency's key activities include scientific research, education, development of anti-doping capacities, and monitoring of the World Anti-Doping Code, whose provisions are enforced by the UNESCO International Convention against Doping in Sport. The aims of the Council of Europe Anti-Doping Convention and the United States Anti-Doping Agency are also closely aligned with those of WADA.

Gene doping is the hypothetical non-therapeutic use of gene therapy by athletes in order to improve their performance in those sporting events which prohibit such applications of genetic modification technology, and for reasons other than the treatment of disease. As of April 2015, there is no evidence that gene doping has been used for athletic performance-enhancement in any sporting events. Gene doping would involve the use of gene transfer to increase or decrease gene expression and protein biosynthesis of a specific human protein; this could be done by directly injecting the gene carrier into the person, or by taking cells from the person, transfecting the cells, and administering the cells back to the person.

Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.
Blood doping is the practice of boosting the number of red blood cells in the bloodstream in order to enhance athletic performance. Because such blood cells carry oxygen from the lungs to the muscles, a higher concentration in the blood can improve an athlete's aerobic capacity (VO2 max) and endurance. Blood doping can be achieved by making the body produce more red blood cells itself using drugs, giving blood transfusions either from another person or back to the same individual, or by using blood substitutes.
This article is about the history of competitors at the Olympic Games using banned athletic performance-enhancing drugs.
Operación Puerto is the code name of a still unfinished Spanish Police operation against the pro sports doping network of Doctor Eufemiano Fuentes. It started in May 2006, which resulted in a scandal that involved several of the world's most famous cyclists and teams at the time.
Performance-enhancing substances, also known as performance-enhancing drugs (PED), are substances that are used to improve any form of activity performance in humans. A well-known example involves doping in sport, where banned physical performance–enhancing drugs are used by athletes and bodybuilders. Athletic performance-enhancing substances are sometimes referred to as ergogenic aids. Cognitive performance-enhancing drugs, commonly called nootropics, are sometimes used by students to improve academic performance. Performance-enhancing substances are also used by military personnel to enhance combat performance.
Cheating at the Paralympic Games has caused scandals that have significantly changed the way in which the International Paralympic Committee (IPC) manages the events.

Erythropoiesis-stimulating agents (ESA) are medications which stimulate the bone marrow to make red blood cells. They are used to treat anemia due to end stage kidney disease, chemotherapy, major surgery, or certain treatments in HIV/AIDS. In these situations they decrease the need for blood transfusions. The different agents are more or less equivalent. They are given by injection.

Methoxy polyethylene glycol-epoetin beta is the active ingredient of a drug marketed by Hoffmann-La Roche under the brand name Mircera. Mircera is a long-acting erythropoietin receptor activator (CERA) indicated for the treatment of patients with anaemia associated with chronic kidney disease. It is the first approved, chemically modified erythropoiesis-stimulating agent (ESA). Mircera is supplied as a solution in pre-filled syringes for intravenous or subcutaneous administration. Mircera was approved for use in Europe in July 2007 by the European Commission, in September 2007 by the Swissmedic, and in November 2007 by the U.S. Food and Drug Administration for use in the United States.

GW501516 is a PPARδ receptor agonist that was invented in a collaboration between Ligand Pharmaceuticals and GlaxoSmithKline in the 1990s, was entered into clinical development as a drug candidate for metabolic diseases and cardiovascular diseases, and was abandoned in 2007 because animal testing showed that the drug caused cancer to develop rapidly in several organs.
Continuous erythropoietin receptor activator (CERA) is the generic term for drugs in a new class of third-generation erythropoiesis-stimulating agents (ESAs). In the media, these agents are commonly referred to as 'EPO', short for erythropoietin. CERAs have an extended half-life and a mechanism of action that promotes increased stimulation of erythropoietin receptors compared with other ESAs.
Don H. Catlin is an anti-doping scientist and one of the founders of modern drug-testing in sport.
An athlete biological passport is an individual electronic record for professional athletes, in which profiles of biological markers of doping and results of doping tests are collated over a period of time. Doping violations can be detected by noting variances from an athlete's established levels outside permissible limits, rather than testing for and identifying illegal substances.
References
- ↑ AdisInsight Erythropoietin gene therapy - Oxford BioMedica Page accessed June 5, 2016
- 1 2 Gretchen Reynolds for The New York Times. June 3, 2007. Outlaw DNA
- ↑ World Anti Doping Agency. (October 2009). Gene Doping. In World Anti-Doping Agency. Retrieved April 11, 2012, from http://www.wada-ama.org/en/Science-Medicine/Science-topics/Gene-Doping/.