
Pleckstrin homology domain or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton.
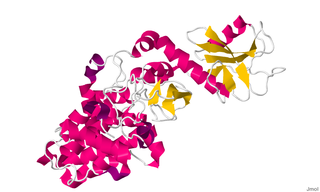
Chimerin 1 (CHN1), also known as alpha-1-chimerin, n-chimerin, is a protein which in humans is encoded by the CHN1 gene.

C1 domain binds an important secondary messenger diacylglycerol (DAG), as well as the analogous phorbol esters. Phorbol esters can directly stimulate protein kinase C, PKC.

Rho GTPase-activating protein 1 is an enzyme that in humans is encoded by the ARHGAP1 gene.
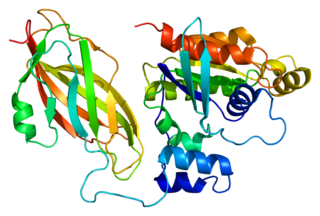
Rac2 is a small signaling G protein, and is a member of the Rac subfamily of the family Rho family of GTPases. It is encoded by the gene RAC2.
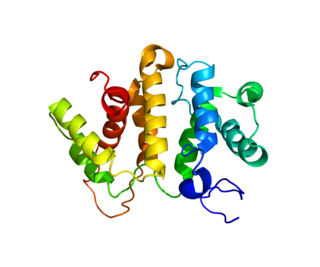
Rho GTPase-activating protein 5 is an enzyme that in humans is encoded by the ARHGAP5 gene.

Rho GTPase-activating protein 4 is an enzyme that in humans is encoded by the ARHGAP4 gene. It has been shown to regulate cell motility and axonal outgrowth in vitro.

Rnd2 is a small signaling G protein, and is a member of the Rnd subgroup of the Rho family of GTPases. It is encoded by the gene RND2.
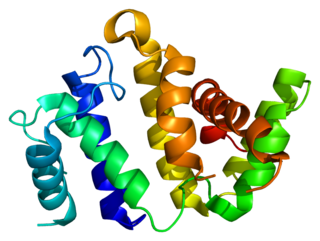
Rho GTPase activating protein 26 (ARHGAP26) also known as GTPase Regulator Associated with Focal Adhesion Kinase (GRAF) is a protein that in humans is encoded by the ARHGAP26 gene.

Rho GTPase-activating protein 8 is a protein that in humans is encoded by the ARHGAP8 gene.
SLIT-ROBO Rho GTPase-activating protein 1 is an enzyme that in humans is encoded by the SRGAP1 gene.

StAR-related lipid transfer domain protein 13 (STARD13) also known as deleted in liver cancer 2 protein (DLC-2) is a protein that in humans is encoded by the STARD13 gene and a member of the DLC family of proteins.

Rho GTPase-activating protein 9 is an enzyme that in humans is encoded by the ARHGAP9 gene.

StAR-related lipid transfer domain protein 8 (STARD8) also known as deleted in liver cancer 3 protein (DLC-3) is a protein that in humans is encoded by the STARD8 gene and is a member of the DLC family.

The Rho GTPase activating protein 31 is encoded in humans by the ARHGAP31 gene. It is a Cdc42/Rac1 GTPase regulator.
Rho GTPase activating protein 18 is a protein that in humans is encoded by the ARHGAP18 gene. The gene is also known as MacGAP and bA307O14.2. ARHGAP18 belongs to a family of Rho GTPase-activating proteins that modulate cell signaling.
ARHGAP29 is a gene located on chromosome 1p22 that encodes Rho GTPase activating protein (GAP) 29, a protein that mediates the cyclical regulation of small GTP binding proteins such as RhoA.

DEP Domain Containing Protein 1B also known as XTP1, XTP8, HBV XAg-Transactivated Protein 8, [formerly referred to as BRCC3] is a human protein encoded by a gene of similar name located on chromosome 5.

ARHGAP11B is a human-specific gene that amplifies basal progenitors, controls neural progenitor proliferation, and contributes to neocortex folding. It is capable of causing neocortex folding in mice. This likely reflects a role for ARHGAP11B in development and evolutionary expansion of the human neocortex, a conclusion consistent with the finding that the gene duplication that created ARHGAP11B occurred on the human lineage after the divergence from the chimpanzee lineage but before the divergence from Neanderthals.

Katrin Rittinger is a professor in structural biology who has made significant contributions to the ubiquitination field. She is a senior scientist at the Francis Crick Institute and was awarded European Molecular Biology Organization (EMBO) membership in 2019. Rittinger is on the Editorial Board of Biochemical Journal and has written on transparency and openness in science.

















