Pathogenicity islands (PAIs), as termed in 1990, are a distinct class of genomic islands acquired by microorganisms through horizontal gene transfer. Pathogenicity islands are found in both animal and plant pathogens. Additionally, PAIs are found in both gram-positive and gram-negative bacteria. They are transferred through horizontal gene transfer events such as transfer by a plasmid, phage, or conjugative transposon. Therefore, PAIs contribute to microorganisms' ability to evolve.

Pseudomonas putida is a Gram-negative, rod-shaped, saprophytic soil bacterium. It has a versatile metabolism and is amenable to genetic manipulation, making it a common organism used in research, bioremediation, and synthesis of chemicals and other compounds.

Micrococcus is a genus of bacteria in the Micrococcaceae family. Micrococcus occurs in a wide range of environments, including water, dust, and soil. Micrococci have Gram-positive spherical cells ranging from about 0.5 to 3 micrometers in diameter and typically appear in tetrads. They are catalase positive, oxidase positive, indole negative and citrate negative. Micrococcus has a substantial cell wall, which may comprise as much as 50% of the cell mass. The genome of Micrococcus is rich in guanine and cytosine (GC), typically exhibiting 65 to 75% GC-content. Micrococci often carry plasmids that provide the organism with useful traits.

Burkholderia cenocepacia is a Gram-negative, rod-shaped bacterium that is commonly found in soil and water environments and may also be associated with plants and animals, particularly as a human pathogen. It is one of over 20 species in the Burkholderia cepacia complex (Bcc) and is notable due to its virulence factors and inherent antibiotic resistance that render it a prominent opportunistic pathogen responsible for life-threatening, nosocomial infections in immunocompromised patients, such as those with cystic fibrosis or chronic granulomatous disease. The quorum sensing systems CepIR and CciIR regulate the formation of biofilms and the expression of virulence factors such as siderophores and proteases. Burkholderia cenocepacia may also cause disease in plants, such as in onions and bananas. Additionally, some strains serve as plant growth-promoting rhizobacteria.
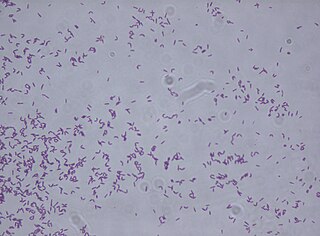
Rhodococcus fascians is a Gram positive bacterial phytopathogen that causes leafy gall disease. R. fascians is the only phytopathogenic member of the genus Rhodococcus; its host range includes both dicotyledonous and monocotyledonous hosts. Because it commonly afflicts tobacco (Nicotiana) plants, it is an agriculturally significant pathogen.
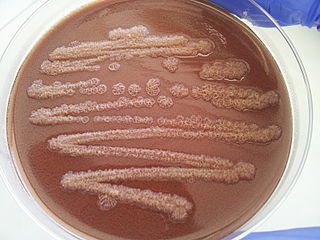
Pseudomonas stutzeri is a Gram-negative soil bacterium that is motile, has a single polar flagellum, and is classified as bacillus, or rod-shaped. While this bacterium was first isolated from human spinal fluid, it has since been found in many different environments due to its various characteristics and metabolic capabilities. P. stutzeri is an opportunistic pathogen in clinical settings, although infections are rare. Based on 16S rRNA analysis, this bacterium has been placed in the P. stutzeri group, to which it lends its name.
Microbial biodegradation is the use of bioremediation and biotransformation methods to harness the naturally occurring ability of microbial xenobiotic metabolism to degrade, transform or accumulate environmental pollutants, including hydrocarbons, polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), heterocyclic compounds, pharmaceutical substances, radionuclides and metals.

Rhodococcus equi is a Gram-positive coccobacillus bacterium. The organism is commonly found in dry and dusty soil and can be important for diseases of domesticated animals. The frequency of infection can reach near 60%. R. equi is an important pathogen causing pneumonia in foals. Since 2008, R. equi has been known to infect wild boar and domestic pigs. R. equi can infect humans. At-risk groups are immunocompromised people, such as HIV-AIDS patients or transplant recipients. Rhodococcus infection in these patients resemble clinical and pathological signs of pulmonary tuberculosis. It is facultative intracellular.
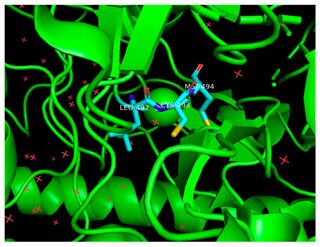
The enzyme benzylsuccinate synthase catalyzes the chemical reaction
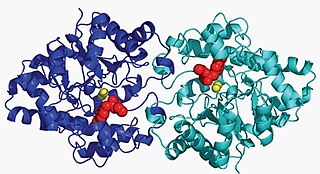
Aryldialkylphosphatase is a metalloenzyme that hydrolyzes the triester linkage found in organophosphate insecticides:
Cupriavidus metallidurans is a non-spore-forming, Gram-negative bacterium which is adapted to survive several forms of heavy metal stress.
Gallaecimonas is a recently described genus of bacteria. The first described species of this genus was Gallaecimonas pentaromativorans gen. nov., sp. nov. isolated by Rodríguez Blanco et al. in 2010 from intertidal sediments of the ria of Corcubión. It is a Gram-negative, rod-shaped, halotolerant bacterium in the class Gammaproteobacteria. It can degrade high molecular mass polycyclic aromatic hydrocarbons of 4 and 5 rings. The 16S rRNA gene sequences of the type strain CEE_131(T) proved to be distantly related to those of Rheinheimera and Serratia. Its G+C content was 41.7 mol%.
Acinetobacter venetianus is a species of bacteria notable for degrading n-alkanes. It harbours plasmids carrying sequences similar to the Pseudomonas oleovorans alkane hydroxylase gene alkBFGH. Its potential for bioremediation is an active research topic, particularly its role in the production of the bioemulsifier emulsan. Its type strain is RAG-1T(=ATCC 31012T=CCUG 45561T=LMG 19082T=LUH 3904T=NIPH 1925T).
Sphingomonas yanoikuyae is a short rod-shaped, strictly aerobic, Gram-negative, non-motile, non-spore-forming, chemoheterotrophic species of bacteria that is yellow or off-white in color. Its type strain is JCM 7371. It is notable for degrading a variety of aromatic compounds including biphenyl, naphthalene, phenanthrene, toluene, m-, and p-xylene. S. yanoikuyae was discovered by Brian Goodman on the southern coast of Papua New Guinea. However, Sphingomonas have a wide distribution across freshwater, seawater, and terrestrial habitats. This is due to the bacteria's ability to grow and survive under low-nutrient conditions as it can utilize a broad range of organic compounds.
Rhodococcus erythropolis is a bacterium species in the genus Rhodococcus. It is Gram-positive. R. erythropolis has been isolated from the air of the Russian Space Laboratory Mir along with a large number of other microorganisms that steadily accumulated during the lifespan of the station. Rhodococcus bacteria are known to degrade organic compounds contained in the rubber used aboard the space station with specialized enzymes. This can lead to degradation of critical components and necessitates replacement of the parts or preventive measures dealing with microbial contamination.
Rhodococcus opacus is a bacterium species in the genus Rhodococcus. It is moderately chemolithotrophic. Its genome has been sequenced. R. opacus possesses extensive catabolic pathways for both sugars and aromatics and can tolerate inhibitory compounds found in depolymerized biomass
Gordonia is a genus of gram-positive, aerobic, catalase-positive bacterium in the Actinomycetota, closely related to the Rhodococcus, Mycobacterium, Skermania, and Nocardia genera. Gordonia bacteria are aerobic, non-motile, and non-sporulating. Gordonia is from the same lineage that includes Mycobacterium tuberculosis. The genus was discovered by Tsukamura in 1971 and named after American bacteriologist Ruth Gordon. Many species are often found in the soil, while other species have been isolated from aquatic environments. Some species have been associated with problems like sludge bulking and foaming in wastewater treatment plants. Gordonia species are rarely known to cause infections in humans.
Novosphingobium aromaticivorans is a species of bacteria. It is an aromatic compound-degrading bacteria, it is gram-negative, non-spore-forming, non-motile and rod-shaped. It is found in deep-terrestrial-subsurface sediments.
Metallosphaera hakonensis is a gram-negative, thermoacidophilic archaea discovered in the hot springs of Hakone National Park, Kanagawa, Japan.
Hydrocarbonoclastic bacteria are a heterogeneous group of prokaryotes which can degrade and utilize hydrocarbon compounds as source of carbon and energy. Despite being present in most of environments around the world, several of these specialized bacteria live in the sea and have been isolated from polluted seawater.












