
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer. The reactive center can be radical, anionic or cationic.
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst in a solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid and gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts.
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have also been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.

In organic chemistry, olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.
A transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene. Carbene complexes have been synthesized from most transition metals and f-block metals, using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction. The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes. Described often as =CR2, carbene ligands are intermediate between alkyls (−CR3) and carbynes (≡CR). Many different carbene-based reagents such as Tebbe's reagent are used in synthesis. They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.
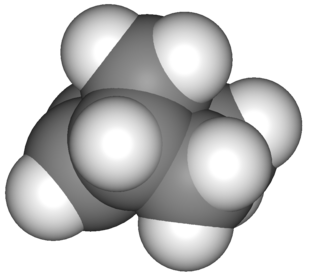
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity.
Dynamic covalent chemistry (DCvC) is a synthetic strategy employed by chemists to make complex molecular and supramolecular assemblies from discrete molecular building blocks. DCvC has allowed access to complex assemblies such as covalent organic frameworks, molecular knots, polymers, and novel macrocycles. Not to be confused with dynamic combinatorial chemistry, DCvC concerns only covalent bonding interactions. As such, it only encompasses a subset of supramolecular chemistries.

Robert Howard GrubbsForMemRS was an American chemist and the Victor and Elizabeth Atkins Professor of Chemistry at the California Institute of Technology in Pasadena, California. He was a co-recipient of the 2005 Nobel Prize in Chemistry for his work on olefin metathesis.

Yves Chauvin was a French chemist and Nobel Prize laureate. He was honorary research director at the Institut français du pétrole and a member of the French Academy of Science. He was known for his work for deciphering the process of olefin metathesis for which he was awarded the 2005 Nobel Prize in Chemistry along with Robert H. Grubbs and Richard R. Schrock.
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.

Herbert S. Eleuterio was an American industrial chemist noted for technical contributions to catalysis, polymerization, industrial research management, and science education. In particular, he discovered the olefin metathesis reaction and several novel fluoropolymers. Additionally, he explored techniques for research leadership, especially methods for fostering collaboration, globalization, and scientific creativity.
Functionalized polyolefins are olefin polymers with polar and nonpolar functionalities attached onto the polymer backbone. There has been an increased interest in functionalizing polyolefins due to their increased usage in everyday life. Polyolefins are virtually ubiquitous in everyday life, from consumer food packaging to biomedical applications; therefore, efforts must be made to study catalytic pathways towards the attachment of various functional groups onto polyolefins in order to affect the material's physical properties.
Janis Louie is a Chemistry professor and Henry Eyring Fellow at The University of Utah. Louie contributes to the chemistry world with her research in inorganic, organic, and polymer chemistry.
Carbonyl olefin metathesis is a type of metathesis reaction that entails, formally, the redistribution of fragments of an alkene and a carbonyl by the scission and regeneration of carbon-carbon and carbon-oxygen double bonds respectively. It is a powerful method in organic synthesis using simple carbonyls and olefins and converting them into less accessible products with higher structural complexity.
Heterogeneous metal catalyzed cross-coupling is a subset of metal catalyzed cross-coupling in which a heterogeneous metal catalyst is employed. Generally heterogeneous cross-coupling catalysts consist of a metal dispersed on an inorganic surface or bound to a polymeric support with ligands. Heterogeneous catalysts provide potential benefits over homogeneous catalysts in chemical processes in which cross-coupling is commonly employed—particularly in the fine chemical industry—including recyclability and lower metal contamination of reaction products. However, for cross-coupling reactions, heterogeneous metal catalysts can suffer from pitfalls such as poor turnover and poor substrate scope, which have limited their utility in cross-coupling reactions to date relative to homogeneous catalysts. Heterogeneous metal catalyzed cross-couplings, as with homogeneous metal catalyzed ones, most commonly use Pd as the cross-coupling metal.
Jennifer Ann Love is an American professor of chemistry at the University of Calgary. She is a Fellow of the Chemical Institute of Canada.







