- RsaA: Secondary structure of RsaA. Rfam family RF01816
- RsaB: Secondary structure of RsaB. Rfam family RF01817
- RsaC: Secondary structure of RsaC. Rfam family RF01818
- RsaD: Secondary structure of RsaD. Rfam family RF01819
- RsaE: Secondary structure of RsaE. Rfam family RF01820
- RsaF: Secondary structure of RsaF. Rfam family RF01858
- RsaG: Secondary structure of RsaG.
- RsaH: Secondary structure of RsaH. Rfam family RF01821
- RsaI: Secondary structure of RsaI. Rfam family RF01775
- RsaJ: Secondary structure of RsaJ. Rfam family RF01822
RsaE
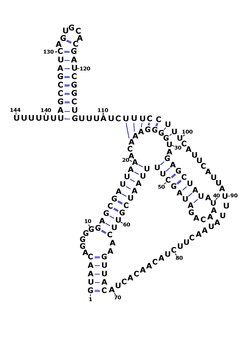
RsaE is found in other members of the genus Staphylococcus such as Staphylococcus epidermidis and Staphylococcus saprophyticus and is the only Rsa RNA to be found outside of this genus, in Macrococcus caseolyticus and Bacillus . In Bacillus subtilis , RsaE had previously been identified as ncr22. [8] [9] RsaE is also consistently found downstream of PepF which codes for oligoendopeptidase F. The function of RsaE was discovered using gene knockout analysis and gene overexpression - it was found to regulate the expression of several enzymes involved in metabolism via antisense binding of their mRNA. [1] [3] [10]
RsaE was shown to be regulated by the presence of nitric oxide (NO). In Bacillus subtilis it controls expression of genes with functions related to oxidative stress and oxidation-reduction reactions and it was renamed RoxS (for related to oxidative stress). [11]









