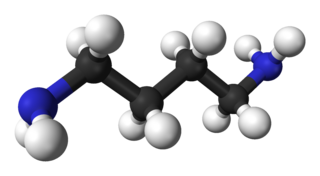
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine. Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors.
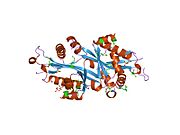
The enzyme ornithine decarboxylase catalyzes the decarboxylation of ornithine to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids and forms a homodimer.
Spermine is a polyamine involved in cellular metabolism that is found in all eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some bacteria as well. It is found as a polycation at physiological pH. Spermine is associated with nucleic acids and is thought to stabilize helical structure, particularly in viruses. It functions as an intracellular free radical scavenger to protect DNA from free radical attack. Spermine is the chemical primarily responsible for the characteristic odor of semen.

Spermidine is a polyamine compound found in ribosomes and living tissues and having various metabolic functions within organisms.

Spermidine synthase is an enzyme that catalyzes the transfer of the propylamine group from S-adenosylmethioninamine to putrescine in the biosynthesis of spermidine. The systematic name is S-adenosyl 3-(methylthio)propylamine:putrescine 3-aminopropyltransferase and it belongs to the group of aminopropyl transferases. It does not need any cofactors. Most spermidine synthases exist in solution as dimers.
Spermine synthase is an enzyme that converts spermidine into spermine. This enzyme catalyses the following chemical reaction
A polyamine oxidase (PAO) is an enzymatic flavoprotein that oxidizes a carbon-nitrogen bond in a secondary amino group of a polyamine donor, using molecular oxygen as an acceptor. The generalized PAO reaction converts three substrates into three products. Different PAOs with varying substrate specificities exist in different organisms. Phylogenetic analyses suggest that PAOs likely evolved once in eukaryotes and diversified by divergent evolution and gene duplication events, though some prokaryotes have acquired PAOs through horizontal gene transfer.
In enzymology, a diamine N-acetyltransferase is an enzyme that catalyzes the chemical reaction

Polyamine-modulated factor 1 is a protein that in humans is encoded by the PMF1 gene.

Spermine oxidase is an enzyme that in humans is encoded by the SMOX gene.

Peroxisomal N(1)-acetyl-spermine/spermidine oxidase is an enzyme that in humans is encoded by the PAOX gene.

Diamine acetyltransferase 2 is an enzyme that in humans is encoded by the SAT2 gene. SAT2 maintains a key metabolic glutamine/glutamate balance underpinning retrograde signaling by dendritic release of the neurotransmitter glutamate.
Keratosis pilaris atropicans is a group of idiopathic genodermatoses that consists of three unique clinical entities: atrophoderma vermiculatum, keratosis follicularis spinulosa decalvans, and keratosis pilaris atrophicans faciei.
Keratosis follicularis spinulosa decalvans is a rare X-linked disorder described by Siemens in 1926. It is a disease that begins in infancy with keratosis pilaris localized on the face, then evolves to more diffuse involvement.
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature.
Spermine oxidase (EC 1.5.3.16, PAOh1/SMO, AtPAO1, AtPAO4, SMO) is an enzyme with systematic name spermidine:oxygen oxidoreductase (spermidine-forming). This enzyme catalyses the following chemical reaction
Non-specific polyamine oxidase (EC 1.5.3.17, polyamine oxidase, Fms1, AtPAO3) is an enzyme with systematic name polyamine:oxygen oxidoreductase (3-aminopropanal or 3-acetamidopropanal-forming). This enzyme catalyses the following chemical reaction
Polyamines (PAs) are small, positively charged, organic molecules that are ubiquitous in all living organisms. These are considered as one of the oldest group of substances known in biochemistry. There are three common types of polyamines, putrescine, spermidine, hermospermine according to structure, universal distribution in all cellular compartments, and presumed involvement in physiological activities. Polyamine is found in all cellular compartments and physiological activities due to their simple structures. The function of polyamine is very diverse in that it performs a key macromolecule to cellular membrane. Because of their essential roles in plant, mutation of polyamines can cause critical damage on plants. Furthermore, some polyamines like putrescine inhibit biosynthetic activities in plants. The activity of polyamines can be categorized to some parts due to its signalling and growing activity.
BpsA is a single-module non-ribosomal peptide synthase (NRPS) located in the cytoplasm responsible for the process of creating branched-chain polyamines, and producing spermidine and spermine. It has a singular ligand in its structure involved with Fe3+ and PLIP interactions. As seen by its EC number, it is a transferase (2) that transfers an alkyl or aryl group other than methyl groups (5) (2.5.1). BpsA was first discovered in the archaea Methanococcus jannaschii and thermophile Thermococcus kodakarensis and since then has been used in a variety of applications such as being used as a reporter, researching phosphopantetheinyl transferase (PPTase), and for NRPS domain recombination experiments it can be used as a model. Both (hyper)thermophilic bacteria and euryarchaeotal archaea seem to conserve BpsA and orthologs as branches chains polyamines are crucial for survival. There is also a second type of BpsA also known as Blue-pigment indigoidine synthetase that produces the pigment indigoidine and is found in organisms like Erwinia chrysanthemi. However, not much seems to be known about this variant except that it is a synthase, and it does not yet appear to be classified under an EC number.