
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

Nitrification is the biological oxidation of ammonia to nitrate via the intermediary nitrite. Nitrification is an important step in the nitrogen cycle in soil. The process of complete nitrification may occur through separate organisms or entirely within one organism, as in comammox bacteria. The transformation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an aerobic process performed by small groups of autotrophic bacteria and archaea.

Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitrification as a type of respiration that reduces oxidized forms of nitrogen in response to the oxidation of an electron donor such as organic matter. The preferred nitrogen electron acceptors in order of most to least thermodynamically favorable include nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O) finally resulting in the production of dinitrogen (N2) completing the nitrogen cycle. Denitrifying microbes require a very low oxygen concentration of less than 10%, as well as organic C for energy. Since denitrification can remove NO3−, reducing its leaching to groundwater, it can be strategically used to treat sewage or animal residues of high nitrogen content. Denitrification can leak N2O, which is an ozone-depleting substance and a greenhouse gas that can have a considerable influence on global warming.

Anammox, an abbreviation for "anaerobic ammonium oxidation", is a globally important microbial process of the nitrogen cycle that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community. In the anammox reaction, nitrite and ammonium ions are converted directly into diatomic nitrogen and water.

A constructed wetland is an artificial wetland to treat sewage, greywater, stormwater runoff or industrial wastewater. It may also be designed for land reclamation after mining, or as a mitigation step for natural areas lost to land development. Constructed wetlands are engineered systems that use the natural functions of vegetation, soil, and organisms to provide secondary treatment to wastewater. The design of the constructed wetland has to be adjusted according to the type of wastewater to be treated. Constructed wetlands have been used in both centralized and decentralized wastewater systems. Primary treatment is recommended when there is a large amount of suspended solids or soluble organic matter.

The activated sludgeprocess is a type of biological wastewater treatment process for treating sewage or industrial wastewaters using aeration and a biological floc composed of bacteria and protozoa. It uses air and microorganisms to biologically oxidize organic pollutants, producing a waste sludge containing the oxidized material.
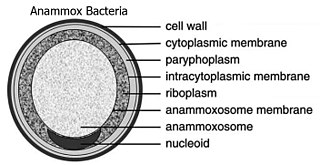
"Candidatus Brocadia anammoxidans" is a bacterial member of the phylum Planctomycetota and therefore lacks peptidoglycan in its cell wall, and has a compartmentalized cytoplasm.
Denitrifying bacteria are a diverse group of bacteria that encompass many different phyla. This group of bacteria, together with denitrifying fungi and archaea, is capable of performing denitrification as part of the nitrogen cycle. Denitrification is performed by a variety of denitrifying bacteria that are widely distributed in soils and sediments and that use oxidized nitrogen compounds such as nitrate and nitrite in the absence of oxygen as a terminal electron acceptor. They metabolize nitrogenous compounds using various enzymes, including nitrate reductase (NAR), nitrite reductase (NIR), nitric oxide reductase (NOR) and nitrous oxide reductase (NOS), turning nitrogen oxides back to nitrogen gas or nitrous oxide.

Secondary treatment is the removal of biodegradable organic matter from sewage or similar kinds of wastewater. The aim is to achieve a certain degree of effluent quality in a sewage treatment plant suitable for the intended disposal or reuse option. A "primary treatment" step often precedes secondary treatment, whereby physical phase separation is used to remove settleable solids. During secondary treatment, biological processes are used to remove dissolved and suspended organic matter measured as biochemical oxygen demand (BOD). These processes are performed by microorganisms in a managed aerobic or anaerobic process depending on the treatment technology. Bacteria and protozoa consume biodegradable soluble organic contaminants while reproducing to form cells of biological solids. Secondary treatment is widely used in sewage treatment and is also applicable to many agricultural and industrial wastewaters.

Sequencing batch reactors (SBR) or sequential batch reactors are a type of activated sludge process for the treatment of wastewater. SBRs treat wastewater such as sewage or output from anaerobic digesters or mechanical biological treatment facilities in batches. Oxygen is bubbled through the mixture of wastewater and activated sludge to reduce the organic matter. The treated effluent may be suitable for discharge to surface waters or possibly for use on land.
Nitrifying bacteria are chemolithotrophic organisms that include species of genera such as Nitrosomonas, Nitrosococcus, Nitrobacter, Nitrospina, Nitrospira and Nitrococcus. These bacteria get their energy from the oxidation of inorganic nitrogen compounds. Types include ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Many species of nitrifying bacteria have complex internal membrane systems that are the location for key enzymes in nitrification: ammonia monooxygenase, hydroxylamine oxidoreductase, and nitrite oxidoreductase.
Simultaneous nitrification–denitrification (SNdN) is a wastewater treatment process. Microbial simultaneous nitrification-denitrification is the conversion of the ammonium ion to nitrogen gas in a single bioreactor. The process is dependent on floc characteristics, reaction kinetics, mass loading of readily biodegradable chemical oxygen demand, rbCOD, and the dissolved oxygen, DO, concentration

A trickling filter is a type of wastewater treatment system. It consists of a fixed bed of rocks, coke, gravel, slag, polyurethane foam, sphagnum peat moss, ceramic, or plastic media over which sewage or other wastewater flows downward and causes a layer of microbial slime (biofilm) to grow, covering the bed of media. Aerobic conditions are maintained by splashing, diffusion, and either by forced-air flowing through the bed or natural convection of air if the filter medium is porous. The treatment of sewage or other wastewater with trickling filters is among the oldest and most well characterized treatment technologies.
CandidatusScalindua wagneri is a Gram-negative coccoid-shaped bacterium that was first isolated from a wastewater treatment plant. This bacterium is an obligate anaerobic chemolithotroph that undergoes anaerobic ammonium oxidation (anammox). It can be used in the wastewater treatment industry in nitrogen reactors to remove nitrogenous wastes from wastewater without contributing to fixed nitrogen loss and greenhouse gas emission.
"Candidatus Scalindua" is a bacterial genus, and a proposed member of the order Planctomycetales. These bacteria lack peptidoglycan in their cell wall and have a compartmentalized cytoplasm. They are ammonium oxidizing bacteria found in marine environments.

The rotating cell biofilm reactor (RCBR) is a new type of biological process based on biofilm active sludge used in wastewaters treatment. RCBR reactors represent an evolution of rotating biological contactors plants: in fact, they are able to develop about ten times the biological surface of rotating biological contactors plants, considering the same biological reactors volume (made of the set of disks). Such a result is obtained using – instead of a set of disks fixed on the central structured tree – many tridimensional plastic elements as carriers. These carriers are used to furnish the necessary biological surface for the cohesion of the bacterial film, responsible of the depuration process. These plastic elements are contained in a cilindric permeable cell, which is like the shape of the reactor cell of a traditional rotating RBC plant. One of the main peculiarity of the RCBR plant is represented by the fact that the biological carriers are common recycled water bottle plastic caps.
Comammox is the name attributed to an organism that can convert ammonia into nitrite and then into nitrate through the process of nitrification. Nitrification has traditionally thought to be a two-step process, where ammonia-oxidizing bacteria and archaea oxidize ammonia to nitrite and then nitrite-oxidizing bacteria convert to nitrate. Complete conversion of ammonia into nitrate by a single microorganism was first predicted in 2006. In 2015 the presence of microorganisms that could carry out both conversion processes was discovered within the genus Nitrospira, and the nitrogen cycle was updated. Within the genus Nitrospira, the major ecosystems comammox are primarily found in natural aquifers and engineered ecosystems.
Dissimilatory nitrate reduction to ammonium (DNRA), also known as nitrate/nitrite ammonification, is the result of anaerobic respiration by chemoorganoheterotrophic microbes using nitrate (NO3−) as an electron acceptor for respiration. In anaerobic conditions microbes which undertake DNRA oxidise organic matter and use nitrate (rather than oxygen) as an electron acceptor, reducing it to nitrite, and then to ammonium (NO3− → NO2− → NH4+).
An oxygen minimum zone (OMZ) is characterized as an oxygen-deficient layer in the world's oceans. Typically found between 200m to 1500m deep below regions of high productivity, such as the western coasts of continents. OMZs can be seasonal following the spring-summer upwelling season. Upwelling of nutrient-rich water leads to high productivity and labile organic matter, that is respired by heterotrophs as it sinks down the water column. High respiration rates deplete the oxygen in the water column to concentrations of 2 mg/L or less forming the OMZ. OMZs are expanding, with increasing ocean deoxygenation. Under these oxygen-starved conditions, energy is diverted from higher trophic levels to microbial communities that have evolved to use other biogeochemical species instead of oxygen, these species include Nitrate, Nitrite, Sulphate etc. Several Bacteria and Archea have adapted to live in these environments by using these alternate chemical species and thrive. The most abundant phyla in OMZs are Pseudomonadota, Bacteroidota, Actinomycetota, and Planctomycetota.
Anammox is a wastewater treatment technique that removes nitrogen using anaerobic ammonium oxidation (anammox). This process is performed by anammox bacteria which are autotrophic, meaning they do not need organic carbon for their metabolism to function. Instead, the metabolism of anammox bacteria convert ammonium and nitrite into dinitrogen gas. Anammox bacteria are a wastewater treatment technique and wastewater treatment facilities are in the process of implementing anammox-based technologies to further enhance ammonia and nitrogen removal.










