Related Research Articles

In biology, the extracellular matrix (ECM), also called intercellular matrix (ICM), is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
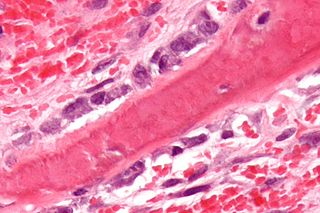
Osteoblasts are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon.
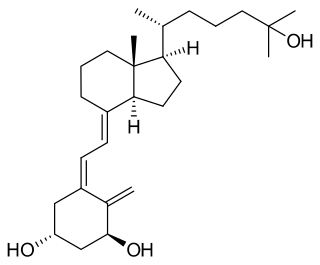
Osteomalacia is a disease characterized by the softening of the bones caused by impaired bone metabolism primarily due to inadequate levels of available phosphate, calcium, and vitamin D, or because of resorption of calcium. The impairment of bone metabolism causes inadequate bone mineralization.

Dentin or dentine is a calcified tissue of the body and, along with enamel, cementum, and pulp, is one of the four major components of teeth. It is usually covered by enamel on the crown and cementum on the root and surrounds the entire pulp. By volume, 45% of dentin consists of the mineral hydroxyapatite, 33% is organic material, and 22% is water. Yellow in appearance, it greatly affects the color of a tooth due to the translucency of enamel. Dentin, which is less mineralized and less brittle than enamel, is necessary for the support of enamel. Dentin rates approximately 3 on the Mohs scale of mineral hardness. There are two main characteristics which distinguish dentin from enamel: firstly, dentin forms throughout life; secondly, dentin is sensitive and can become hypersensitive to changes in temperature due to the sensory function of odontoblasts, especially when enamel recedes and dentin channels become exposed.
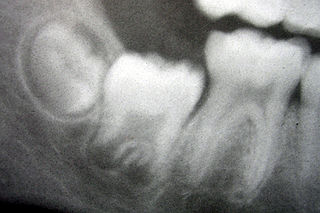
Tooth development or odontogenesis is the complex process by which teeth form from embryonic cells, grow, and erupt into the mouth. For human teeth to have a healthy oral environment, all parts of the tooth must develop during appropriate stages of fetal development. Primary (baby) teeth start to form between the sixth and eighth week of prenatal development, and permanent teeth begin to form in the twentieth week. If teeth do not start to develop at or near these times, they will not develop at all, resulting in hypodontia or anodontia.

Osteopontin (OPN), also known as bone /sialoprotein I, early T-lymphocyte activation (ETA-1), secreted phosphoprotein 1 (SPP1), 2ar and Rickettsia resistance (Ric), is a protein that in humans is encoded by the SPP1 gene. The murine ortholog is Spp1. Osteopontin is a SIBLING (glycoprotein) that was first identified in 1986 in osteoblasts.

X-linked hypophosphatemia (XLH) is an X-linked dominant form of rickets that differs from most cases of dietary deficiency rickets in that vitamin D supplementation does not cure it. It can cause bone deformity including short stature and genu varum (bow-leggedness). It is associated with a mutation in the PHEX gene sequence (Xp.22) and subsequent inactivity of the PHEX protein. PHEX mutations lead to an elevated circulating (systemic) level of the hormone FGF23 which results in renal phosphate wasting, and local elevations of the mineralization/calcification-inhibiting protein osteopontin in the extracellular matrix of bones and teeth. An inactivating mutation in the PHEX gene results in an increase in systemic circulating FGF23, and a decrease in the enzymatic activity of the PHEX enzyme which normally removes (degrades) mineralization-inhibiting osteopontin protein; in XLH, the decreased PHEX enzyme activity leads to an accumulation of inhibitory osteopontin locally in bones and teeth to block mineralization which, along with renal phosphate wasting, both cause osteomalacia and odontomalacia.

Osteonectin (ON) also known as secreted protein acidic and rich in cysteine (SPARC) or basement-membrane protein 40 (BM-40) is a protein that in humans is encoded by the SPARC gene.

Bone sialoprotein (BSP) is a component of mineralized tissues such as bone, dentin, cementum and calcified cartilage. BSP is a significant component of the bone extracellular matrix and has been suggested to constitute approximately 8% of all non-collagenous proteins found in bone and cementum. BSP, a SIBLING protein, was originally isolated from bovine cortical bone as a 23-kDa glycopeptide with high sialic acid content.
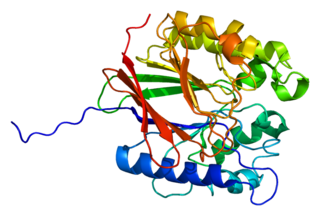
Tartrate-resistant acid phosphatase, also called acid phosphatase 5, tartrate resistant (ACP5), is a glycosylated monomeric metalloprotein enzyme expressed in mammals. It has a molecular weight of approximately 35kDa, a basic isoelectric point (7.6–9.5), and optimal activity in acidic conditions. TRAP is synthesized as latent proenzyme and activated by proteolytic cleavage and reduction. It is differentiated from other mammalian acid phosphatases by its resistance to inhibition by tartrate and by its molecular weight.

Phosphate-regulating endopeptidase homolog X-linked also known as phosphate-regulating gene with homologies to endopeptidases on the X chromosome or metalloendopeptidase homolog PEX is an enzyme that in humans is encoded by the PHEX gene. This gene contains 18 exons and is located on the X chromosome.
Biomimetic materials are materials developed using inspiration from nature. This may be useful in the design of composite materials. Natural structures have inspired and innovated human creations. Notable examples of these natural structures include: honeycomb structure of the beehive, strength of spider silks, bird flight mechanics, and shark skin water repellency. The etymological roots of the neologism "biomimetic" derive from Greek, since bios means "life" and mimetikos means "imitative".

Dentin matrix acidic phosphoprotein 1 is a protein that in humans is encoded by the DMP1 gene.

Extracellular matrix protein 1 is a protein that in humans is encoded by the ECM1 gene.

Matrix extracellular phosphoglycoprotein is a protein that in humans is encoded by the MEPE gene. A conserved RGD motif is found in this protein, and this is potentially involved in integrin recognition.

Family with sequence similarity 20, member C also known as FAM20C or DMP4 is a protein which in humans is encoded by the FAM20C gene. Fam20C, a Golgi localized protein kinase, is a serine kinase that phosphorylates both casein and other highly acidic proteins and members of the small integrin-binding ligand, the N-linked glycoproteins (SIBLING) family at the target motif SerXGlu.
Dentin sialophosphoprotein is a precursor protein for other proteins found in the teeth. It is produced by cells (odontoblasts) inside the teeth, and in smaller quantities by bone tissues. It is required for normal hardening (mineralisation) of teeth. During teeth development, it is broken down into three proteins such as dentin sialoprotein (DSP), dentin glycoprotein (DGP), and dentin phosphoprotein (DPP). These proteins become the major non-collagenous components of teeth. Their distribution in the collagen matrix of the forming dentin suggests these proteins play an important role in the regulation of mineral deposition. Additional evidence for this correlation is phenotypically manifested in patients with mutant forms of dentin sialophosphoprotein. Such patients suffer dental anomalies including type III dentinogenesis imperfecta.
Dentin sialoprotein is a protein found in teeth. It is one of the two proteins produced by the segmentation of dentin sialophosphoprotein. Dentin sialoprotein can be found in the dentin immediately subjacent to cellular cementum, but not subjacent to acellular fibrous cementum.

Mineralized tissues are biological tissues that incorporate minerals into soft matrices. Typically these tissues form a protective shield or structural support. Bone, mollusc shells, deep sea sponge Euplectella species, radiolarians, diatoms, antler bone, tendon, cartilage, tooth enamel and dentin are some examples of mineralized tissues.
The human skeletal system is a complex organ in constant equilibrium with the rest of the body. In addition to supporting and giving structure to the body, a bone is the major reservoir for many minerals and compounds essential for maintaining a healthy pH balance. The deterioration of the body with age renders the elderly particularly susceptible to and affected by poor bone health. Illnesses like osteoporosis, characterized by weakening of the bone's structural matrix, increases the risk of hip-fractures and other life-changing secondary symptoms. In 2010, over 258,000 people aged 65 and older were admitted to the hospital for hip fractures. Incidence of hip fractures is expected to rise by 12% in America, with a projected 289,000 admissions in the year 2030. Other sources estimate up to 1.5 million Americans will have an osteoporotic-related fracture each year. The cost of treating these people is also enormous, in 1991 Medicare spent an estimated $2.9 billion for treatment and out-patient care of hip fractures, this number can only be expected to rise.
References
- 1 2 Qin, C.; Baba, O.; Butler, W.T. (2004). "Post-Translational Modifications of Sibling Proteins and Their Roles in Osteogenesis and Dentinogenesis". Critical Reviews in Oral Biology & Medicine. 15 (3): 126–36. doi:10.1177/154411130401500302. PMID 15187031.
- ↑ Chaplet, M; De Leval, L; Waltregny, D; Detry, C; Fornaciari, G; Bevilacqua, G; Fisher, LW; Castronovo, V; Bellahcène, A (2003). "Dentin Matrix Protein 1 is Expressed in Human Lung Cancer". Journal of Bone and Mineral Research. 18 (8): 1506–12. doi: 10.1359/jbmr.2003.18.8.1506 . PMID 12929940. S2CID 24870810. INIST 15005341.
- ↑ Rittling, Susan R.; Denhardt, David T. (1999). "Osteopontin Function in Pathology: Lessons from Osteopontin-Deficient Mice". Nephron Experimental Nephrology. 7 (2): 103–13. doi:10.1159/000020591. PMID 10213864. S2CID 19785030.