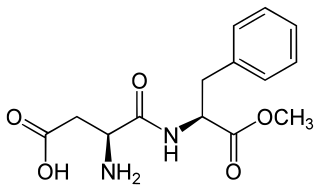
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose and is commonly used as a sugar substitute in foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide with brand names NutraSweet, Equal, and Canderel. Aspartame was approved by the US Food and Drug Administration (FDA) in 1974, and then again in 1981, after approval was revoked in 1980.

Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salting), smoke (smoking), sugar (crystallization), etc. This allows for longer-lasting foods such as bacon, sweets or wines. With the advent of ultra-processed foods in the second half of the twentieth century, many additives have been introduced, of both natural and artificial origin. Food additives also include substances that may be introduced to food indirectly in the manufacturing process, through packaging, or during storage or transport.

Stevia is a sweet sugar substitute that is about 50 to 300 times sweeter than sugar. It is extracted from the leaves of Stevia rebaudiana, a plant native to areas of Paraguay and Brazil in the southern Amazon rainforest. The active compounds in stevia are steviol glycosides. Stevia is heat-stable, pH-stable, and not fermentable. Humans cannot metabolize the glycosides in stevia, and therefore it has zero calories. Its taste has a slower onset and longer duration than that of sugar, and at high concentrations some of its extracts may have an aftertaste described as licorice-like or bitter. Stevia is used in sugar- and calorie-reduced food and beverage products as an alternative for variants with sugar.

Sucralose is an artificial sweetener and sugar substitute. As the majority of ingested sucralose is not metabolized by the body, it adds no calories. In the European Union, it is also known under the E number E955. It is produced by chlorination of sucrose, selectively replacing three of the hydroxy groups—in the C1 and C6 positions of the fructose portion and the C4 position of the glucose portion—to give a 1,6-dichloro-1,6-dideoxyfructose–4-chloro-4-deoxygalactose disaccharide. Sucralose is about 600 times sweeter than sucrose, three times as sweet as both aspartame and acesulfame potassium, and twice as sweet as sodium saccharin.

A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar substitute products are commercially available in various forms, such as small pills, powders, and packets.

Cyclamate is an artificial sweetener. It is 30–50 times sweeter than sucrose, making it the least potent of the commercially used artificial sweeteners. It is often used with other artificial sweeteners, especially saccharin; the mixture of 10 parts cyclamate to 1 part saccharin is common and masks the off-tastes of both sweeteners. It is less expensive than most sweeteners, including sucralose, and is stable under heating. Safety concerns led to it being banned in a few countries, though the European Union considers it safe.

Saccharin, also called saccharine or benzosulfimide, or used in saccharin sodium or saccharin calcium forms, is a non-nutritive artificial sweetener. Saccharin is a benzoic sulfimide that is about 500 times sweeter than sucrose, but has a bitter or metallic aftertaste, especially at high concentrations. It is used to sweeten products, such as drinks, candies, baked goods, tobacco products, excipients, and for masking the bitter taste of some medicines. It appears as white crystals and is odorless.

Acesulfame potassium, also known as acesulfame K or Ace K, is a synthetic calorie-free sugar substitute often marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number E950. It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG. In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C
4H
4KNO
4S and a molecular weight of 201.24 g/mol.

Diet or light beverages are generally sugar-free, artificially sweetened beverages with few or no calories. They are marketed for diabetics and other people who want to reduce their sugar and/or caloric intake.

Tab was a diet cola soft drink produced and distributed by The Coca-Cola Company, introduced in 1963 and discontinued in 2020. The company's first diet drink, Tab was popular among some people throughout the 1960s and 1970s as an alternative to Coca-Cola. Several variations were made, including a number of fruit-flavored, root beer, and ginger ale versions. Caffeine-free and clear variations were released in the late 1980s and early 1990s.

The Food Additives Amendment of 1958 is a 1958 amendment to the United States' Food, Drugs, and Cosmetic Act of 1938. It was a response to concerns about the safety of new food additives. The amendment established an exemption from the "food additive" definition and requirements for substances "generally recognized as safe" by scientific experts in the field, based on long history of use before 1958 or based on scientific studies. New food additives would be subject to testing including by the "Delaney clause". The Delaney clause was a provision in the amendment which said that if a substance were found to cause cancer in man or animal, then it could not be used as a food additive.

The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) is a United States federal law that set up the basic U.S. system of pesticide regulation to protect applicators, consumers, and the environment. It is administered and regulated by the United States Environmental Protection Agency (EPA) and the appropriate environmental agencies of the respective states. FIFRA has undergone several important amendments since its inception. A significant revision in 1972 by the Federal Environmental Pesticide Control Act (FEPCA) and several others have expanded EPA's present authority to oversee the sales and use of pesticides with emphasis on the preservation of human health and protection of the environment by "(1) strengthening the registration process by shifting the burden of proof to the chemical manufacturer, (2) enforcing compliance against banned and unregistered products, and (3) promulgating the regulatory framework missing from the original law".
The International Agency for Research on Cancer is an intergovernmental agency forming part of the World Health Organization of the United Nations. Its role is to conduct and coordinate research into the causes of cancer. It also collects and publishes surveillance data regarding the occurrence of cancer worldwide.

2-Butoxyethanol is an organic compound with the chemical formula BuOC2H4OH (Bu = CH3CH2CH2CH2). This colorless liquid has a sweet, ether-like odor, as it derives from the family of glycol ethers, and is a butyl ether of ethylene glycol. As a relatively nonvolatile, inexpensive solvent, it is used in many domestic and industrial products because of its properties as a surfactant. It is a known respiratory irritant and can be acutely toxic, but animal studies did not find it to be mutagenic, and no studies suggest it is a human carcinogen. A study of 13 classroom air contaminants conducted in Portugal reported a statistically significant association with increased rates of nasal obstruction and a positive association below the level of statistical significance with a higher risk of obese asthma and increased child BMI.

Potassium bromate is a bromate of potassium and takes the form of white crystals or powder. It is a strong oxidizing agent.
The artificial sweetener aspartame has been the subject of several controversies since its initial approval by the U.S. Food and Drug Administration (FDA) in 1974. The FDA approval of aspartame was highly contested, beginning with suspicions of its involvement in brain cancer, alleging that the quality of the initial research supporting its safety was inadequate and flawed, and that conflicts of interest marred the 1981 approval of aspartame, previously evaluated by two FDA panels that concluded to keep the approval on hold before further investigation. In 1987, the U.S. Government Accountability Office concluded that the food additive approval process had been followed properly for aspartame. The irregularities fueled a conspiracy theory, which the "Nancy Markle" email hoax circulated, along with claims—counter to the weight of medical evidence—that numerous health conditions are caused by the consumption of aspartame in normal doses.

Signed into effect on 12 June 2002, the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (PHSBPRA) was signed by the President, the Department of Health and Human Services (DHHS) and the U.S. Department of Agriculture (USDA).

Food contact materials or food contacting substances (FCS) are materials that are intended to be in contact with food. These can be things that are quite obvious like a glass or a can for soft drinks as well as machinery in a food factory or a coffee machine.

The regulation of food and dietary supplements by the U.S. Food and Drug Administration is a process governed by various statutes enacted by the United States Congress and interpreted by the U.S. Food and Drug Administration ("FDA"). Pursuant to the Federal Food, Drug, and Cosmetic Act and accompanying legislation, the FDA has authority to oversee the quality of substances sold as food in the United States, and to monitor claims made in the labeling about both the composition and the health benefits of foods.

Pesticide regulation in the United States is primarily a responsibility of the Environmental Protection Agency (EPA). In America, it was not till the 1950s that pesticides were regulated in terms of their safety. The Pesticides Control Amendment (PCA) of 1954 was the first time Congress passed guidance regarding the establishment of safe limits for pesticide residues on food. It authorized the Food and Drug Administration (FDA) to ban pesticides they determined to be unsafe if they were sprayed directly on food. The Food Additives Amendment, which included the Delaney Clause, prohibited the pesticide residues from any carcinogenic pesticides in processed food. In 1959, pesticides were required to be registered.



















