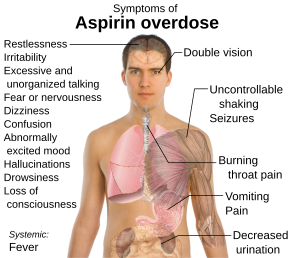
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease, pericarditis, and rheumatic fever.
Iron poisoning typically occurs from ingestion of excess iron that results in acute toxicity. Mild symptoms which occur within hours include vomiting, diarrhea, abdominal pain, and drowsiness. In more severe cases, symptoms can include tachypnea, low blood pressure, seizures, or coma. If left untreated, iron poisoning can lead to multi-organ failure resulting in permanent organ damage or death.
Diuresis is the excretion of urine, especially when excessive (polyuria). The term collectively denotes the physiologic processes underpinning increased urine production by the kidneys during maintenance of fluid balance.

Acetazolamide, sold under the trade name Diamox among others, is a medication used to treat glaucoma, epilepsy, acute mountain sickness, periodic paralysis, idiopathic intracranial hypertension, heart failure and to alkalinize urine. It may be used long term for the treatment of open angle glaucoma and short term for acute angle closure glaucoma until surgery can be carried out. It is taken by mouth or injection into a vein. Acetazolamide is a first generation carbonic anhydrase inhibitor and it decreases the ocular fluid and osmolality in the eye to decrease intraocular pressure.
Acidosis is a process causing increased acidity in the blood and other body tissues. If not further qualified, it usually refers to acidity of the blood plasma.

Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the body's acid-base balance. Metabolic acidosis has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids. Metabolic acidosis can lead to acidemia, which is defined as arterial blood pH that is lower than 7.35. Acidemia and acidosis are not mutually exclusive – pH and hydrogen ion concentrations also depend on the coexistence of other acid-base disorders; therefore, pH levels in people with metabolic acidosis can range from low to high.

Respiratory acidosis is a state in which decreased ventilation (hypoventilation) increases the concentration of carbon dioxide in the blood and decreases the blood's pH.

Nicotine poisoning describes the symptoms of the toxic effects of nicotine following ingestion, inhalation, or skin contact. Nicotine poisoning can potentially be deadly, though serious or fatal overdoses are rare. Historically, most cases of nicotine poisoning have been the result of use of nicotine as an insecticide. More recent cases of poisoning typically appear to be in the form of Green Tobacco Sickness, or due to unintended ingestion of tobacco or tobacco products or consumption of nicotine-containing plants.

Respiratory alkalosis is a medical condition in which increased respiration elevates the blood pH beyond the normal range (7.35–7.45) with a concurrent reduction in arterial levels of carbon dioxide. This condition is one of the four primary disturbance of acid–base homeostasis.

Metabolic alkalosis is a metabolic condition in which the pH of tissue is elevated beyond the normal range (7.35–7.45). This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations. The condition typically cannot last long if the kidneys are functioning properly.
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases in the ECF is crucial for the normal physiology of the body—and for cellular metabolism. The pH of the intracellular fluid and the extracellular fluid need to be maintained at a constant level.
Ethylene glycol poisoning is poisoning caused by drinking ethylene glycol. Early symptoms include intoxication, vomiting and abdominal pain. Later symptoms may include a decreased level of consciousness, headache, and seizures. Long term outcomes may include kidney failure and brain damage. Toxicity and death may occur after drinking even in a small amount as ethylene glycol is more toxic than other diols.
Paracetamol poisoning, also known as acetaminophen poisoning, is caused by excessive use of the medication paracetamol (acetaminophen). Most people have few or non-specific symptoms in the first 24 hours following overdose. These symptoms include feeling tired, abdominal pain, or nausea. This is typically followed by absence of symptoms for a couple of days, after which yellowish skin, blood clotting problems, and confusion occurs as a result of liver failure. Additional complications may include kidney failure, pancreatitis, low blood sugar, and lactic acidosis. If death does not occur, people tend to recover fully over a couple of weeks. Without treatment, death from toxicity occurs 4 to 18 days later.

High anion gap metabolic acidosis is a form of metabolic acidosis characterized by a high anion gap. Metabolic acidosis occurs when the body produces too much acid, or when the kidneys are not removing enough acid from the body. Several types of metabolic acidosis occur, grouped by their influence on the anion gap.

Tricyclic antidepressant overdose is poisoning caused by excessive medication of the tricyclic antidepressant (TCA) type. Symptoms may include elevated body temperature, blurred vision, dilated pupils, sleepiness, confusion, seizures, rapid heart rate, and cardiac arrest. If symptoms have not occurred within six hours of exposure they are unlikely to occur.
The Hs and Ts is a mnemonic used to aid in remembering the possible reversible causes of cardiac arrest. A variety of disease processes can lead to a cardiac arrest; however, they usually boil down to one or more of the "Hs and Ts".

Barbiturate overdose is poisoning due to excessive doses of barbiturates. Symptoms typically include difficulty thinking, poor coordination, decreased level of consciousness, and a decreased effort to breathe. Complications of overdose can include noncardiogenic pulmonary edema. If death occurs this is typically due to a lack of breathing.

Intravenous sodium bicarbonate, also known as sodium hydrogen carbonate, is a medication primarily used to treat severe metabolic acidosis. For this purpose it is generally only used when the pH is less than 7.1 and when the underlying cause is either diarrhea, vomiting, or the kidneys. Other uses include high blood potassium, tricyclic antidepressant overdose, and cocaine toxicity as well as a number of other poisonings. It is given by injection into a vein.

Methanol toxicity is poisoning from methanol, characteristically via ingestion. Symptoms may include a decreased level of consciousness, poor or no coordination, vomiting, abdominal pain, and a specific smell on the breath. Decreased vision may start as early as twelve hours after exposure. Long-term outcomes may include blindness and kidney failure. Blindness may occur after drinking as little as 10 mL; death may occur after drinking quantities over 15 mL.

Lithium toxicity, also known as lithium overdose, is the condition of having too much lithium. Symptoms may include a tremor, increased reflexes, trouble walking, kidney problems, and an altered level of consciousness. Some symptoms may last for a year after levels return to normal. Complications may include serotonin syndrome.