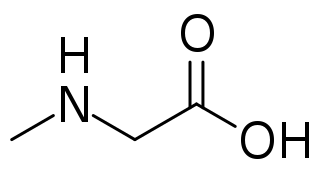
Sarcosine, also known as N-methylglycine, or monomethylglycine, is a amino acid with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine.

Irwin Allan Rose was an American biologist. Along with Aaron Ciechanover and Avram Hershko, he was awarded the 2004 Nobel Prize in Chemistry for the discovery of ubiquitin-mediated protein degradation.
Acyl-CoA dehydrogenases (ACADs) are a class of enzymes that function to catalyze the initial step in each cycle of fatty acid β-oxidation in the mitochondria of cells. Their action results in the introduction of a trans double-bond between C2 (α) and C3 (β) of the acyl-CoA thioester substrate. Flavin adenine dinucleotide (FAD) is a required co-factor in addition to the presence of an active site glutamate in order for the enzyme to function.

Serine hydroxymethyltransferase (SHMT) is a pyridoxal phosphate (PLP) (Vitamin B6) dependent enzyme (EC 2.1.2.1) which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine and tetrahydrofolate (THF) to 5,10-Methylenetetrahydrofolate (5,10-CH2-THF). This reaction provides the largest part of the one-carbon units available to the cell.
17β-Hydroxysteroid dehydrogenases, also 17-ketosteroid reductases (17-KSR), are a group of alcohol oxidoreductases which catalyze the reduction of 17-ketosteroids and the dehydrogenation of 17β-hydroxysteroids in steroidogenesis and steroid metabolism. This includes interconversion of DHEA and androstenediol, androstenedione and testosterone, and estrone and estradiol.

L-Arginine:glycine amidinotransferase is the enzyme that catalyses the transfer of an amidino group from L-arginine to glycine. The products are L-ornithine and glycocyamine, also known as guanidinoacetate, the immediate precursor of creatine. Creatine and its phosphorylated form play a central role in the energy metabolism of muscle and nerve tissues. Creatine is in highest concentrations in the skeletal muscle, heart, spermatozoa and photoreceptor cells. Creatine helps buffer the rapid changes in ADP/ATP ratio in muscle and nerve cells during active periods. Creatine is also synthesized in other tissues, such as pancreas, kidneys, and liver, where amidinotransferase is located in the cytoplasm, including the intermembrane space of the mitochondria, of the cells that make up those tissues.
In enzymology, a testosterone 17beta-dehydrogenase is an enzyme that catalyzes the chemical reaction between testosterone and androst-4-ene-3,17-dione. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor.
In enzymology, a dihydropyrimidine dehydrogenase (NADP+) (EC 1.3.1.2) is an enzyme that catalyzes the chemical reaction

In enzymology, an isovaleryl-CoA dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a D-lactate dehydrogenase (cytochrome) is an enzyme that catalyzes the chemical reaction

In enzymology, a 3-methyl-2-oxobutanoate dehydrogenase (EC 1.2.4.4) is an enzyme that catalyzes the chemical reaction

In enzymology, a betaine-aldehyde dehydrogenase (EC 1.2.1.8) is an enzyme that catalyzes the chemical reaction
In enzymology, a dimethylglycine dehydrogenase (EC 1.5.8.4) is an enzyme that catalyzes the chemical reaction

Glycine decarboxylase also known as glycine cleavage system P protein or glycine dehydrogenase is an enzyme that in humans is encoded by the GLDC gene.
In enzymology, a nitroalkane oxidase (EC 1.7.3.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a trimethylamine dehydrogenase (EC 1.5.8.2) is an enzyme that catalyzes the chemical reaction

In enzymology, a 3-oxoacid CoA-transferase is an enzyme that catalyzes the chemical reaction
Glutaminolysis (glutamine + -lysis) is a series of biochemical reactions by which the amino acid glutamine is lysed to glutamate, aspartate, CO2, pyruvate, lactate, alanine and citrate.
The glycine cleavage system (GCS) is also known as the glycine decarboxylase complex or GDC. The system is a series of enzymes that are triggered in response to high concentrations of the amino acid glycine. The same set of enzymes is sometimes referred to as glycine synthase when it runs in the reverse direction to form glycine. The glycine cleavage system is composed of four proteins: the T-protein, P-protein, L-protein, and H-protein. They do not form a stable complex, so it is more appropriate to call it a "system" instead of a "complex". The H-protein is responsible for interacting with the three other proteins and acts as a shuttle for some of the intermediate products in glycine decarboxylation. In both animals and plants, the glycine cleavage system is loosely attached to the inner membrane of the mitochondria. Mutations in this enzymatic system are linked with glycine encephalopathy.

NADH:ubiquinone reductase (non-electrogenic) (EC 1.6.5.9, NDH-2, ubiquinone reductase, coenzyme Q reductase, dihydronicotinamide adenine dinucleotide-coenzyme Q reductase, DPNH-coenzyme Q reductase, DPNH-ubiquinone reductase, NADH-coenzyme Q oxidoreductase, NADH-coenzyme Q reductase, NADH-CoQ oxidoreductase, NADH-CoQ reductase) is an enzyme with systematic name NADH:ubiquinone oxidoreductase. This enzyme catalyses the following chemical reaction:
















