
Botrytis cinerea is a necrotrophic fungus that affects many plant species, although its most notable hosts may be wine grapes. In viticulture, it is commonly known as "botrytis bunch rot"; in horticulture, it is usually called "grey mould" or "gray mold".

Texas root rot is a disease that is fairly common in Mexico and the southwestern United States resulting in sudden wilt and death of affected plants, usually during the warmer months. It is caused by a soil-borne fungus named Phymatotrichopsis omnivora that attacks the roots of susceptible plants. It was first discovered in 1888 by Pammel and later named by Duggar in 1916.

Aspergillus flavus is a saprotrophic and pathogenic fungus with a cosmopolitan distribution. It is best known for its colonization of cereal grains, legumes, and tree nuts. Postharvest rot typically develops during harvest, storage, and/or transit. Its specific name flavus derives from the Latin meaning yellow, a reference to the frequently observed colour of the spores. A. flavus infections can occur while hosts are still in the field (preharvest), but often show no symptoms (dormancy) until postharvest storage or transport. In addition to causing preharvest and postharvest infections, many strains produce significant quantities of toxic compounds known as mycotoxins, which, when consumed, are toxic to mammals. A. flavus is also an opportunistic human and animal pathogen, causing aspergillosis in immunocompromised individuals.

A sclerotium, is a compact mass of hardened fungal mycelium containing food reserves. One role of sclerotia is to survive environmental extremes. In some higher fungi such as ergot, sclerotia become detached and remain dormant until favorable growth conditions return. Sclerotia initially were mistaken for individual organisms and described as separate species until Louis René Tulasne proved in 1853 that sclerotia are only a stage in the life cycle of some fungi. Further investigation showed that this stage appears in many fungi belonging to many diverse groups. Sclerotia are important in the understanding of the life cycle and reproduction of fungi, as a food source, as medicine, and in agricultural blight management.

Rhizoctonia solani is a species of fungus in the order Cantharellales. Basidiocarps are thin, effused, and web-like, but the fungus is more typically encountered in its anamorphic state, as hyphae and sclerotia. The name Rhizoctonia solani is currently applied to a complex of related species that await further research. In its wide sense, Rhizoctonia solani is a facultative plant pathogen with a wide host range and worldwide distribution. It causes various plant diseases such as root rot, damping off, and wire stem. It can also form mycorrhizal associations with orchids.

Gibberella zeae, also known by the name of its anamorph Fusarium graminearum, is a fungal plant pathogen which causes fusarium head blight (FHB), a devastating disease on wheat and barley. The pathogen is responsible for billions of dollars in economic losses worldwide each year. Infection causes shifts in the amino acid composition of wheat, resulting in shriveled kernels and contaminating the remaining grain with mycotoxins, mainly deoxynivalenol (DON), which inhibits protein biosynthesis; and zearalenone, an estrogenic mycotoxin. These toxins cause vomiting, liver damage, and reproductive defects in livestock, and are harmful to humans through contaminated food. Despite great efforts to find resistance genes against F. graminearum, no completely resistant variety is currently available. Research on the biology of F. graminearum is directed towards gaining insight into more details about the infection process and reveal weak spots in the life cycle of this pathogen to develop fungicides that can protect wheat from scab infection.

Macrophomina phaseolina is a Botryosphaeriaceae plant pathogen fungus that causes damping off, seedling blight, collar rot, stem rot, charcoal rot, basal stem rot, and root rot on many plant species.
Mycoleptodiscus terrestris is a fungal plant pathogen.
Pythium aphanidermatum is a soil borne plant pathogen. Pythium is a genus in the class Oomycetes, which are also known as water molds. Oomycetes are not true fungi, as their cell walls are made of cellulose instead of chitin, they are diploid in their vegetative state, and they form coenocytic hyphae. Also, they reproduce asexually with motile biflagelette zoospores that require water to move towards and infect a host. Sexually, they reproduce with structures called antheridia, oogonia, and oospores.
Magnaporthe salvinii is a fungus known to attack a variety of grass and rice species, including Oryza sativa and Zizania aquatica. Symptoms of fungal infection in plants include small, black, lesions on the leaves that develop into more widespread leaf rot, which then spreads to the stem and causes breakage. As part of its life cycle, the fungus produces sclerotia that persist in dead plant tissue and the soil. Management of the fungus may be effected by tilling the soil, reducing its nitrogen content, or by open field burning, all of which reduce the number of sclerotia, or by the application of a fungicide.
Sclerotinia minor is a plant pathogen infecting Chicory, Radicchio, carrots, tomatoes, sunflowers, peanuts and lettuce.

Diaporthe phaseolorum var. sojae is a plant pathogen infecting soybean and peanut.
Phialophora gregata is a Deuteromycete fungus that is a plant pathogen which causes the disease commonly known as brown stem rot of soybean. P. gregata does not produce survival structures, but has the ability to overwinter as mycelium in decaying soybean residue.
This article summarizes different crops, what common fungal problems they have, and how fungicide should be used in order to mitigate damage and crop loss. This page also covers how specific fungal infections affect crops present in the United States.

Helicobasidium is a genus of fungi in the subdivision Pucciniomycotina. Basidiocarps are corticioid (patch-forming) and are typically violet to purple. Microscopically they have auricularioid basidia. Asexual anamorphs, formerly referred to the genus Thanatophytum, produce sclerotia. Conidia-bearing anamorphs are parasitic on rust fungi and are currently still referred to the genus Tuberculina.
Stromatinia cepivora is a fungus in the division Ascomycota. It is the teleomorph of Sclerotium cepivorum, the cause of white rot in onions, garlic, and leeks. The infective sclerotia remain viable in the soil for many years and are stimulated to germinate by the presence of a susceptible crop.

Collar rot is a symptomatically described disease that is usually caused by any one of various fungal and oomycete plant pathogens. It is present where the pathogen causes a lesion localized at or about the collet between the stem and the root. The lesions develop around the stem eventually forming a "collar". Observationally, collar rot grades into "basal stem rot", and with some pathogens is the first phase of "basal stem rot" often followed by "root rot". Collar rot is most often observed in seedings grown in infected soil. The pathogens that cause collar rot may be species or genera specific. But generalist pathogens such as Agroathelia rolfsii are known to attack over 200 different species. While bacteria caused collar rot is not common, trees infected with Fire blight may develop collar rot. Non-parasitic collar rot may be caused by winter damage.
Stem rot is a disease caused by a fungus infection in the stem of crop plants. Fungus that causes stem rot are in the Rhizoctonia, Fusarium or Pythium genera. Stem rot can readily infect crops that are in their vegetative or flowering stages. The disease can survive up to five years in the soil. Symptoms of stem rot includes staining of infected area, reduced crop yield and crop failure. The disease can be spread through the use of unfiltered water as well as unsterilized tools. Also leaving previous dead roots in soil can increase the risk of stem rot. Spores can also enter the plant through injured stem tissue on the plant including from insect attacks. The fungus impedes stem functions like transporting nutrients. It can cause water to leak through the lesions of stem tissue. Common infected crop plants are soybeans and potatoes. An issue with maintaining this disease is the lack of management by crop producers. Producers of soybeans tend to not manage for the disease because it is not normally yield limiting in a large area. Fungicides can be used to manage the disease as well as burning the crop after harvest or letting it decompose.

Elisabeth Eirian Jones is a New Zealand phytopathologist, and a full professor at Lincoln University, specialising in sustainable control strategies for cropping industries.
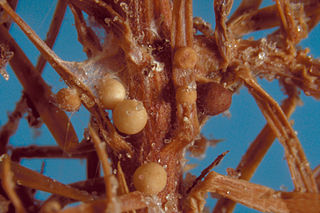
Agroathelia rolfsii is a corticioid fungus in the order Amylocorticiales. It is a facultative plant pathogen and is the causal agent of "southern blight" disease in crops.















