Related Research Articles

Corundum is a crystalline form of aluminium oxide typically containing traces of iron, titanium, vanadium, and chromium. It is a rock-forming mineral. It is a naturally transparent material, but can have different colors depending on the presence of transition metal impurities in its crystalline structure. Corundum has two primary gem varieties: ruby and sapphire. Rubies are red due to the presence of chromium, and sapphires exhibit a range of colors depending on what transition metal is present. A rare type of sapphire, padparadscha sapphire, is pink-orange.
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick.

The Mohs scale of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of minerals through the ability of harder material to scratch softer material.

Lapidary is the practice of shaping stone, minerals, or gemstones into decorative items such as cabochons, engraved gems, and faceted designs. A person who practices lapidary is known as a lapidarist. A lapidarist uses the lapidary techniques of cutting, grinding, and polishing. Hardstone carving requires specialized carving techniques.
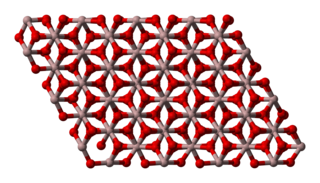
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is used to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Carl Friedrich Christian Mohs was a German chemist and mineralogist. He was the creator of the Mohs scale of mineral hardness. Mohs also introduced a classification of the crystal forms in crystal systems independently of Christian Samuel Weiss.
An abrasive is a material, often a mineral, that is used to shape or finish a workpiece through rubbing which leads to part of the workpiece being worn away by friction. While finishing a material often means polishing it to gain a smooth, reflective surface, the process can also involve roughening as in satin, matte or beaded finishes. In short, the ceramics which are used to cut, grind and polish other softer materials are known as abrasives.

Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond.

Grinding wheels are wheels that contain abrasive compounds for grinding and abrasive machining operations. Such wheels are also used in grinding machines.

Diamond-like carbon (DLC) is a class of amorphous carbon material that displays some of the typical properties of diamond. DLC is usually applied as coatings to other materials that could benefit from such properties.

Aggregated diamond nanorods, or ADNRs, are a nanocrystalline form of diamond, also known as nanodiamond or hyperdiamond.
In materials science, hardness is a measure of the resistance to localized plastic deformation, such as an indentation or a scratch (linear), induced mechanically either by pressing or abrasion. In general, different materials differ in their hardness; for example hard metals such as titanium and beryllium are harder than soft metals such as sodium and metallic tin, or wood and common plastics. Macroscopic hardness is generally characterized by strong intermolecular bonds, but the behavior of solid materials under force is complex; therefore, hardness can be measured in different ways, such as scratch hardness, indentation hardness, and rebound hardness. Hardness is dependent on ductility, elastic stiffness, plasticity, strain, strength, toughness, viscoelasticity, and viscosity. Common examples of hard matter are ceramics, concrete, certain metals, and superhard materials, which can be contrasted with soft matter.

A diamond tool is a cutting tool with diamond grains fixed on the functional parts of the tool via a bonding material or another method. As diamond is a superhard material, diamond tools have many advantages as compared with tools made with common abrasives such as corundum and silicon carbide.
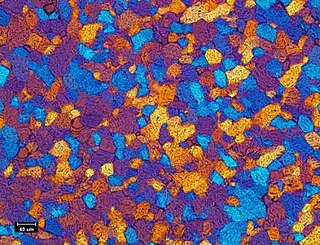
Ceramography is the art and science of preparation, examination and evaluation of ceramic microstructures. Ceramography can be thought of as the metallography of ceramics. The microstructure is the structure level of approximately 0.1 to 100 μm, between the minimum wavelength of visible light and the resolution limit of the naked eye. The microstructure includes most grains, secondary phases, grain boundaries, pores, micro-cracks and hardness microindentations. Most bulk mechanical, optical, thermal, electrical and magnetic properties are significantly affected by the microstructure. The fabrication method and process conditions are generally indicated by the microstructure. The root cause of many ceramic failures is evident in the microstructure. Ceramography is part of the broader field of materialography, which includes all the microscopic techniques of material analysis, such as metallography, petrography and plastography. Ceramography is usually reserved for high-performance ceramics for industrial applications, such as 85–99.9% alumina (Al2O3) in Fig. 1, zirconia (ZrO2), silicon carbide (SiC), silicon nitride (Si3N4), and ceramic-matrix composites. It is seldom used on whiteware ceramics such as sanitaryware, wall tiles and dishware.

Ultrasonic machining is a subtractive manufacturing process that removes material from the surface of a part through high frequency, low amplitude vibrations of a tool against the material surface in the presence of fine abrasive particles. The tool travels vertically or orthogonal to the surface of the part at amplitudes of 0.05 to 0.125 mm. The fine abrasive grains are mixed with water to form a slurry that is distributed across the part and the tip of the tool. Typical grain sizes of the abrasive material range from 100 to 1000, where smaller grains produce smoother surface finishes.

Abrasion is the process of scuffing, scratching, wearing down, marring, or rubbing away. It can be intentionally imposed in a controlled process using an abrasive. Abrasion can be an undesirable effect of exposure to normal use or exposure to the elements.
The Rosiwal scale is a hardness scale in mineralogy, with its name given in memory of the Austrian geologist August Karl Rosiwal. The Rosiwal scale attempts to give more quantitative values of scratch hardness, unlike the Mohs scale which is a qualitative measurement with relative values.
Titanium adhesive bonding is an engineering process used in the aerospace industry, medical-device manufacture and elsewhere. Titanium alloy is often used in medical and military applications because of its strength, weight, and corrosion resistance characteristics. In implantable medical devices, titanium is used because of its biocompatibility and its passive, stable oxide layer. Also, titanium allergies are rare and in those cases mitigations like Parylene coating are used. In the aerospace industry titanium is often bonded to save cost, touch times, and the need for mechanical fasteners. In the past, Russian submarines' hulls were completely made of titanium because the non-magnetic nature of the material went undetected by the defense technology at that time. Bonding adhesive to titanium requires preparing the surface beforehand, and there is not a single solution for all applications. For example, etchant and chemical methods are not biocompatible and cannot be employed when the device will come into contact with blood and tissue. Mechanical surface roughness techniques like sanding and laser roughening may make the surface brittle and create micro-hardness regions that would not be suitable for cyclic loading found in military applications. Air oxidation at high temperatures will produce a crystalline oxide layer at a lower investment cost, but the increased temperatures can deform precision parts. The type of adhesive, thermosetting or thermoplastic, and curing methods are also factors in titanium bonding because of the adhesive's interaction with the treated oxide layer. Surface treatments can also be combined. For example, a grit blast process can be followed by a chemical etch and a primer application.

White fused alumina was obtained by the crystallization of the molten Bayer alumina feedstock in an electric arc furnace. It is commonly called white fused aluminum oxide and may also be called white corundum. The manufacture of white fused alumina is resulting in γ–Al2O3 in industrial alumina powder being completely transformed to α–Al2O3. White fused alumina is white particles or a white powder, and its microstructure is transparent crystal.
References
- ↑ George F. Vander Voort (1999). Metallography, Principles and Practice. ASM International. pp. 368–369. ISBN 9781615032365.
- ↑ Akono, A-T.; P. Reis; F-J. Ulm. (2011). "Scratching as a Fracture Process: From Butter to Steel" (PDF). Physical Review Letters . 106 (20). American Physical Society: 2. Bibcode:2011PhRvL.106t4302A. doi:10.1103/PhysRevLett.106.204302. hdl:1721.1/65375. PMID 21668232. S2CID 1176619 . Retrieved 2022-05-03.
We present results of a hybrid experimental and theoretical investigation of the fracture scaling in scratch tests and show that scratching is a fracture dominated process. Validated for paraffin wax, cement paste, Jurassic limestone and steel, we derive a model that provides a quantitative means to relate quantities measured in scratch tests to fracture properties of materials at multiple scales. The scalability of scratching for different probes and depths opens new venues towards miniaturization of our technique, to extract fracture properties of materials at even smaller length scales.
- ↑ von Groth, Paul Heinrich (1926). Entwicklungsgeschichte der Mineralogischen Wissenschaften [History of the development of the mineralogical sciences] (in German). Berlin: Springer. p. 250. ISBN 9783662409107.
In demselben Jahre (1812) wurde MOHS als Professor am Joanneum angestellt und veröffentliche den ersten Teil seines Werkes "Versuch einer Elementarmethode zur naturhistorischen Bestimmung und Erkennung der Fossilien", in welcher die bekannte Härteskala aufgestellt wurde.
[In the same year (1812) MOHS was employed as a professor at the Joanneum and published the first part of his work "Attempt at an elementary method for the natural-historical determination and recognition of fossils", in which the well-known hardness scale was set up.] - ↑ "Mohs hardness" in Encyclopædia Britannica Online
- ↑ David Tabor (1951). The Hardness of Metals. OUP Oxford. ISBN 978-0-19-850776-5 . Retrieved 2022-05-03.
- 1 2 Industrial Minerals and Rocks: (nonmetallics Other Than Fuels). American Institute of Mining, Metallurgical, and Petroleum Engineers. 1960.
The Mohs scale is inadequate both because the methods of testing are very crude and because the intervals between steps in the scale are not uniform.
- ↑ Kevin J. Anderson (1994). "Hardness Testing" (PDF). MRS Bulletin . Historical Note. 19 (November 1994): 7. doi:10.1557/S0883769400048491 . Retrieved 2022-04-21.
For all its usefulness, the Mohs scale is arbitrary and nonlinear. ... When synthetic abrasive materials become widely available at the beginning of this century, R.R. Ridgway and his co-workers, finding they needed more numbers at the high end of the scale, modified Mohs' scheme. C.E. Wooddell measured how much various minerals resisted wearing down with diamond abrasives, which allowed a finer categorization between the Mohs numbers of 9 and 10. Ridgway arbitrarily shifted the value of diamond to 15 on the scale instead of 10, which allowed them to assign hardness numbers of 12 to fused alumina, 13 to silicon carbide, and 14 to boron carbide.
- ↑ Ridgway, Raymond R; Ballard, Archibald H; Bailey, Bruce L. (1933). "Hardness Values for Electrochemical Products". Transactions of the Electrochemical Society . 63: 369. doi:10.1149/1.3493827 . Retrieved 2022-04-22.
- ↑ Wooddell, Charles E. (1935). "Method of Comparing the Hardness of Electric Furnace Products and Natural Abrasives". Transactions of the Electrochemical Society . 68: 111–130. doi: 10.1149/1.3493860 . Retrieved 2022-04-22.
- ↑ Henry Chandler (1963). "Industrial Diamond : A Materials Survey". Information Circular (8200). United States Department of the Interior: 6–7. Retrieved 2022-05-03.
- ↑ Plendl, Johannes N.; Gielisse, Peter J. (1 Feb 1962). "Hardness of Nonmetallic Solids on an Atomic Basis". Physical Review . 125 (3): 828–832. Bibcode:1962PhRv..125..828P. doi:10.1103/PhysRev.125.828 . Retrieved 2022-04-22.