
Psychrophiles or cryophiles are extremophilic organisms that are capable of growth and reproduction in low temperatures, ranging from −20 °C (−4 °F) to 20 °C (68 °F). They are found in places that are permanently cold, such as the polar regions and the deep sea. They can be contrasted with thermophiles, which are organisms that thrive at unusually high temperatures, and mesophiles at intermediate temperatures. Psychrophile is Greek for 'cold-loving', from Ancient Greek ψυχρός (psukhrós) 'cold, frozen'.

A polynya is an area of open water surrounded by sea ice. It is now used as a geographical term for an area of unfrozen seawater within otherwise contiguous pack ice or fast ice. It is a loanword from the Russian полынья, which refers to a natural ice hole and was adopted in the 19th century by polar explorers to describe navigable portions of the sea.
Ice algae are any of the various types of algal communities found in annual and multi-year sea, and terrestrial lake ice or glacier ice.
An oligotroph is an organism that can live in an environment that offers very low levels of nutrients. They may be contrasted with copiotrophs, which prefer nutritionally rich environments. Oligotrophs are characterized by slow growth, low rates of metabolism, and generally low population density. Oligotrophic environments are those that offer little to sustain life. These environments include deep oceanic sediments, caves, glacial and polar ice, deep subsurface soil, aquifers, ocean waters, and leached soils.

A brine pool, sometimes called an underwater lake, deepwater or brine lake, is a volume of brine collected in a seafloor depression. These pools are dense bodies of water that have a salinity that is typically three to eight times greater than the surrounding ocean. Brine pools are commonly found below polar sea ice and in the deep ocean. This below-sea ice forms through a process called brine rejection. For deep-sea brine pools, salt is necessary to increase the salinity gradient. The salt can come from one of two processes: the dissolution of large salt deposits through salt tectonics or geothermally-heated brine issued from tectonic spreading centers.
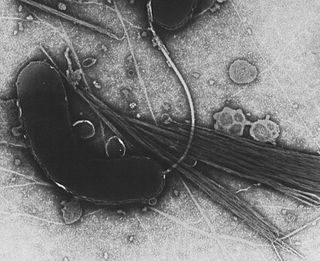
Gammaproteobacteria is a class of bacteria in the phylum Pseudomonadota. It contains about 250 genera, which makes it the most genus-rich taxon of the Prokaryotes. Several medically, ecologically, and scientifically important groups of bacteria belong to this class. All members of this class are Gram-negative. It is the most phylogenetically and physiologically diverse class of the Pseudomonadota.
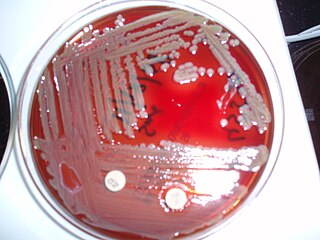
The class Flavobacteriia is composed of a single class of environmental bacteria. It contains the family Flavobacteriaceae, which is the largest family in the phylum Bacteroidota. This class is widely distributed in soil, fresh, and seawater habitats. The name is often spelt Flavobacteria, but was officially named Flavobacteriia in 2012.

In the deep ocean, marine snow is a continuous shower of mostly organic detritus falling from the upper layers of the water column. It is a significant means of exporting energy from the light-rich photic zone to the aphotic zone below, which is referred to as the biological pump. Export production is the amount of organic matter produced in the ocean by primary production that is not recycled (remineralised) before it sinks into the aphotic zone. Because of the role of export production in the ocean's biological pump, it is typically measured in units of carbon. The term was coined by explorer William Beebe as observed from his bathysphere. As the origin of marine snow lies in activities within the productive photic zone, the prevalence of marine snow changes with seasonal fluctuations in photosynthetic activity and ocean currents. Marine snow can be an important food source for organisms living in the aphotic zone, particularly for organisms that live very deep in the water column.
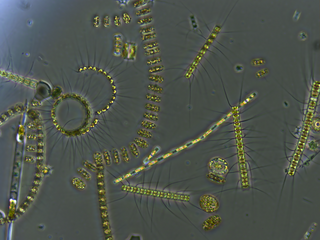
Bacterioplankton refers to the bacterial component of the plankton that drifts in the water column. The name comes from the Ancient Greek word πλαγκτός (planktós), meaning "wandering" or "drifting", and bacterium, a Latin term coined in the 19th century by Christian Gottfried Ehrenberg. They are found in both seawater and fresh water.

Blood Falls is an outflow of an iron(III) oxide–tainted plume of saltwater, flowing from the tongue of Taylor Glacier onto the ice-covered surface of West Lake Bonney in the Taylor Valley of the McMurdo Dry Valleys in Victoria Land, East Antarctica.

Frost flowers are ice crystals commonly found growing on young sea ice and thin lake ice in cold, calm conditions. The ice crystals are similar to hoar frost, and are commonly seen to grow in patches around 3–4 cm in diameter. Frost flowers growing on sea ice have extremely high salinities and concentrations of other sea water chemicals and, because of their high surface area, are efficient releasers of these chemicals into the atmosphere.
Brine rejection is a process that occurs when salty water freezes. The salts do not fit in the crystal structure of water ice, so the salt is expelled.
Polaribacter is a genus in the family Flavobacteriaceae. They are gram-negative, aerobic bacteria that can be heterotrophic, psychrophilic or mesophilic. Most species are non-motile and species range from ovoid to rod-shaped. Polaribacter forms yellow- to orange-pigmented colonies. They have been mostly adapted to cool marine ecosystems, and their optimal growth range is at a temperature between 10 and 32 °C and at a pH of 7.0 to 8.0. They are oxidase and catalase-positive and are able to grow using carbohydrates, amino acids, and organic acids.
Bacterioplankton counting is the estimation of the abundance of bacterioplankton in a specific body of water, which is useful information to marine microbiologists. Various counting methodologies have been developed over the years to determine the number present in the water being observed. Methods used for counting bacterioplankton include epifluorescence microscopy, flow cytometry, measures of productivity through frequency of dividing cells (FDC), thymidine incorporation, and leucine incorporation.

The viral shunt is a mechanism that prevents marine microbial particulate organic matter (POM) from migrating up trophic levels by recycling them into dissolved organic matter (DOM), which can be readily taken up by microorganisms. The DOM recycled by the viral shunt pathway is comparable to the amount generated by the other main sources of marine DOM.

The hydrothermal vent microbial community includes all unicellular organisms that live and reproduce in a chemically distinct area around hydrothermal vents. These include organisms in the microbial mat, free floating cells, or bacteria in an endosymbiotic relationship with animals. Chemolithoautotrophic bacteria derive nutrients and energy from the geological activity at Hydrothermal vents to fix carbon into organic forms. Viruses are also a part of the hydrothermal vent microbial community and their influence on the microbial ecology in these ecosystems is a burgeoning field of research.
Saprotrophic bacteria are bacteria that are typically soil-dwelling and utilize saprotrophic nutrition as their primary energy source. They are often associated with soil fungi that also use saprotrophic nutrition and both are classified as saprotrophs.
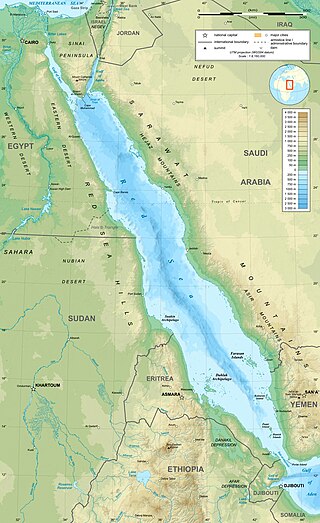
The Red Sea and its extensions of the Gulf of Suez and the Gulf of Aqaba contain the largest recorded concentration of deep-sea brine pools on the planet. These pools have many features that make them uninhabitable to almost all organisms on the planet, yet certain communities of microbes thrive within these extreme environments that have temperatures ranging from 2.0 °C to 75 °C. The Red Sea brine pools have extreme salt concentrations and varying compositions of nutrients and other chemicals that directly affect their microbiomes. There are approximately 25 individual pools in the region, some of which are closely clustered together in groups, leading to their undetermined classification of names. The brine pools originate from hydrothermal vents, the shifting of tectonic plates, and the accumulation of water with properties that make it unsuitable for mixing, leading to its accumulation within faults and divots in the sea floor. Atlantis II Deep, Discovery Deep, and the Kebrit are the most investigated and researched brine pools within the Red Sea. Additionally, many microbial species form beneficial symbiotic relationships with organisms living and feeding in proximity to the pools. These relationships allow for the study of specialized adaptations of microbes to brine pool environments.
A sea ice brine pocket is an area of fluid sea water with a high salt concentration trapped in sea ice as it freezes. Due to the nature of their formation, brine pockets are most commonly found in areas below −2 °C (28 °F), where it is sufficiently cold for seawater to freeze and form sea ice. Though the high salinity and low light conditions of brine pockets create a challenging environment for marine mammals, brine pockets serve as a habitat for various microbes. Sampling and studying these pockets requires specialized equipment to accommodate the hypersaline conditions and subzero temperatures.

An intertidal bioflim is a biofilm that forms on the intertidal region of bodies of water. Bacteria and various microorganisms, including algae and fungi, form communities of adhered cells called biofilms. A matrix of extracellular polymeric substances (EPS) within the biofilm forms sticky coatings on individual sediment particles and detrital surfaces. This feature protects bacteria against environmental stresses like temperature and pH fluctuations, UV exposure, changes in salinity, depletion of nutrients, antimicrobial agents, desiccation, and predation. Particularly, in the ever-changing environments of intertidal systems, biofilms can facilitate a range of microbial processes and create protective microenvironments where cells communicate with each other and regulate further biofilm formation via Quorum Sensing (QS)., While biofilm formation is advantageous to bacteria and other microorganisms involved, the attachment of microorganisms to ship hulls can increase fuel consumption and emission of greenhouse gases, as well as introduce Non-Indigenous Species (NIS), potentially resulting in harmful economic and ecological impacts on the receiving ecosystems.















