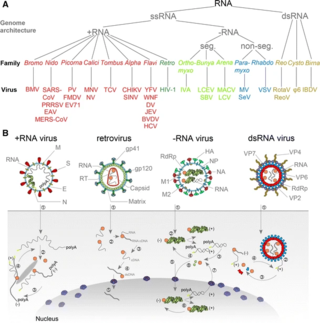
An RNA virus is a virus—other than a retrovirus—that has ribonucleic acid (RNA) as its genetic material. The nucleic acid is usually single-stranded RNA (ssRNA) but it may be double-stranded (dsRNA). Notable human diseases caused by RNA viruses include the common cold, influenza, SARS, MERS, Covid-19, Dengue Virus, hepatitis C, hepatitis E, West Nile fever, Ebola virus disease, rabies, polio, mumps, and measles.

Filoviridae is a family of single-stranded negative-sense RNA viruses in the order Mononegavirales. Two members of the family that are commonly known are Ebola virus and Marburg virus. Both viruses, and some of their lesser known relatives, cause severe disease in humans and nonhuman primates in the form of viral hemorrhagic fevers.

A polyribosome is a group of ribosomes bound to an mRNA molecule like “beads” on a “thread”. It consists of a complex of an mRNA molecule and two or more ribosomes that act to translate mRNA instructions into polypeptides. Originally coined "ergosomes" in 1963, they were further characterized by Jonathan Warner, Paul M. Knopf, and Alex Rich.
Indiana vesiculovirus, formerly Vesicular stomatitis Indiana virus is a virus in the family Rhabdoviridae; the well-known Rabies lyssavirus belongs to the same family. VSIV can infect insects, cattle, horses and pigs. It has particular importance to farmers in certain regions of the world where it infects cattle. This is because its clinical presentation is identical to the very important foot and mouth disease virus.

Rhabdoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates, invertebrates, plants, fungi and protozoans serve as natural hosts. Diseases associated with member viruses include rabies encephalitis caused by the rabies virus, and flu-like symptoms in humans caused by vesiculoviruses. The name is derived from Ancient Greek rhabdos, meaning rod, referring to the shape of the viral particles. The family has 40 genera, most assigned to three subfamilies.

Defective interfering particles (DIPs), also known as defective interfering viruses, are spontaneously generated virus mutants in which a critical portion of the particle's genome has been lost due to defective replication or non-homologous recombination. The mechanism of their formation is presumed to be as a result of template-switching during replication of the viral genome, although non-replicative mechanisms involving direct ligation of genomic RNA fragments have also been proposed. DIPs are derived from and associated with their parent virus, and particles are classed as DIPs if they are rendered non-infectious due to at least one essential gene of the virus being lost or severely damaged as a result of the defection. A DIP can usually still penetrate host cells, but requires another fully functional virus particle to co-infect a cell with it, in order to provide the lost factors.
Pseudotyping is the process of producing viruses or viral vectors in combination with foreign viral envelope proteins. The result is a pseudotyped virus particle, also called a pseudovirus. With this method, the foreign viral envelope proteins can be used to alter host tropism or increase or decrease the stability of the virus particles. Pseudotyped particles do not carry the genetic material to produce additional viral envelope proteins, so the phenotypic changes cannot be passed on to progeny viral particles. In some cases, the inability to produce viral envelope proteins renders the pseudovirus replication incompetent. In this way, the properties of dangerous viruses can be studied in a lower risk setting.
Alice S. Huang (simplified Chinese: 黄诗厚; traditional Chinese: 黃詩厚; pinyin: Huáng Shīhòu; Wade–Giles: Huang Shih-hou; is an American biologist specialized in microbiology and virology. She served as President of AAAS during the 2010–2011 term.

Marburg virus (MARV) is a hemorrhagic fever virus of the Filoviridae family of viruses and a member of the species Marburg marburgvirus, genus Marburgvirus. It causes Marburg virus disease in primates, a form of viral hemorrhagic fever. The virus is considered to be extremely dangerous. The World Health Organization (WHO) rates it as a Risk Group 4 Pathogen. In the United States, the National Institute of Allergy and Infectious Diseases ranks it as a Category A Priority Pathogen and the Centers for Disease Control and Prevention lists it as a Category A Bioterrorism Agent. It is also listed as a biological agent for export control by the Australia Group.

Zaire ebolavirus, more commonly known as Ebola virus, is one of six known species within the genus Ebolavirus. Four of the six known ebolaviruses, including EBOV, cause a severe and often fatal hemorrhagic fever in humans and other mammals, known as Ebola virus disease (EVD). Ebola virus has caused the majority of human deaths from EVD, and was the cause of the 2013–2016 epidemic in western Africa, which resulted in at least 28,646 suspected cases and 11,323 confirmed deaths.

Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-ZEBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. As of 2022, there are only vaccines against the Zaire ebolavirus. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.
David Mahan Knipe is the Higgins Professor of Microbiology and Molecular Genetics in the Department of Microbiology at the Harvard Medical School in Boston, Massachusetts and co-chief editor of the reference book Fields Virology. He returned to the Chair of the Program in Virology at Harvard Medical School in 2019, having previously held the position from 2004 through 2016 and served as interim Co-Chair of the Microbiology and Immunobiology Department from 2016 through 2018.
Judith Alice Lesnaw is an American virologist, photographer, and inductee of the University of Kentucky's Hall of Fame. She was the first woman hired into Biology, the first woman to be tenured, and the first molecular biologist at the University of Kentucky.
Negative-strand RNA viruses are a group of related viruses that have negative-sense, single-stranded genomes made of ribonucleic acid (RNA). They have genomes that act as complementary strands from which messenger RNA (mRNA) is synthesized by the viral enzyme RNA-dependent RNA polymerase (RdRp). During replication of the viral genome, RdRp synthesizes a positive-sense antigenome that it uses as a template to create genomic negative-sense RNA. Negative-strand RNA viruses also share a number of other characteristics: most contain a viral envelope that surrounds the capsid, which encases the viral genome, −ssRNA virus genomes are usually linear, and it is common for their genome to be segmented.
Jack D. Keene is a James B. Duke Professor of Molecular Genetics and Microbiology at Duke University.

Andrea Marzi is a German-American virologist. She is chief of the immunobiology and molecular virology unit at the Rocky Mountain Laboratories. Marzi investigates the pathogenesis of filoviruses and vaccine development. She received the Loeffler-Frosch medal in recognition of her research.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.
Endothelial cell tropism or endotheliotropism is a type of tissue tropism or host tropism that characterizes an pathogen's ability to recognize and infect an endothelial cell. Pathogens, such as viruses, can target a specific tissue type or multiple tissue types. Like other cells, the endothelial cell possesses several features that supports a productive viral infection a cell including, cell surface receptors, immune responses, and other virulence factors. Endothelial cells are found in various tissue types such as in the capillaries, veins, and arteries in the human body. As endothelial cells line these blood vessels and critical networks that extend access to various human organ systems, the virus entry into these cells can be detrimental to virus spread across the host system and affect clinical course of disease. Understanding the mechanisms of how viruses attach, enter, and control endothelial functions and host responses inform infectious disease understanding and medical countermeasures.
Jay Clark Brown is an American molecular biologist, microbiologist, virologist, and academic. He is a Professor Emeritus in the Department of microbiology, immunology, and cancer biology at the University of Virginia School of Medicine.













