
Uranium is a chemical element with symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.

Depleted uranium is uranium with a lower content of the fissile isotope 235
U
than natural uranium. Natural uranium contains about 0.72% 235
U
, while the DU used by the U.S. Department of Defense contains 0.3% 235
U
or less. The less radioactive and non-fissile 238
U
constitutes the main component of depleted uranium. Uses of DU take advantage of its very high density of 19.1 grams per cubic centimetre (0.69 lb/cu in), 68.4% denser than lead.
Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238, uranium-235, and uranium-234. 235U is the only nuclide existing in nature that is fissile with thermal neutrons.
Gore is a town in western Sequoyah County, Oklahoma, United States. It is part of the Fort Smith, Arkansas-Oklahoma Metropolitan Statistical Area. The population was 977 at the 2010 census, an increase of 15 percent over the figure of 850 recorded in 2000.
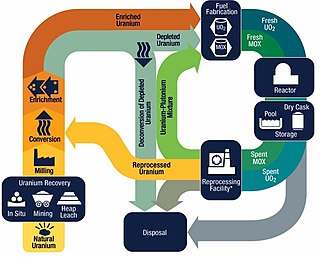
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
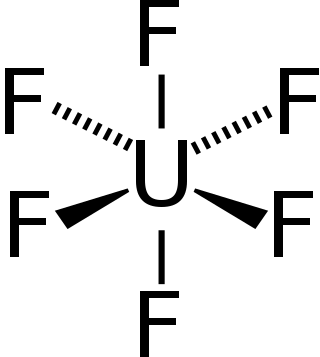
Uranium hexafluoride (UF6), (sometimes called "hex") is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.

The Kerr-McGee Corporation, founded in 1929, was an American energy company involved in oil exploration, production of crude oil, natural gas, perchlorate and uranium mining and milling in various countries. On June 23, 2006, Anadarko Petroleum acquired Kerr-McGee in an all-cash transaction totaling $16.5 billion plus $2.6 billion in debt and all operations moved from their base in Oklahoma, United States.

Gaseous diffusion is a technology that was used to produce enriched uranium by forcing gaseous uranium hexafluoride (UF6) through microporous membranes. This produces a slight separation (enrichment factor 1.0043) between the molecules containing uranium-235 (235U) and uranium-238 (238U). By use of a large cascade of many stages, high separations can be achieved. It was the first process to be developed that was capable of producing enriched uranium in industrially useful quantities, but is nowadays considered obsolete, having been superseded by the more-efficient gas centrifuge process.


The Honeywell Uranium Hexafluoride Processing Facility, a uranium conversion facility, is located 1.9 miles (3 km) northwest of Metropolis, Illinois, United States. The plant, Honeywell Specialty Chemicals in Metropolis, Illinois, has a nominal capacity of 15,000 tU as uranium hexafluoride per year. ConverDyn, a general partnership between affiliates of Honeywell and General Atomics, is the exclusive agent for conversion sales from the Honeywell Uranium Hexafluoride Processing Facility.
Uranium in the environment is a global health concern, and comes from both natural and man-made sources. Mining, phosphates in agriculture, weapons manufacturing, and nuclear power are sources of uranium in the environment.
Uranium mining in New Mexico was a significant industry from the early 1950s until the early 1980s. Although New Mexico has the second largest identified uranium ore reserves of any state in the United States, no uranium ore has been mined in New Mexico since 1998.

The Church Rock uranium mill spill occurred in the U.S. state of New Mexico on July 16, 1979, when United Nuclear Corporation's tailings disposal pond at its uranium mill in Church Rock breached its dam. The accident remains the largest release of radioactive material in U.S. history, having released more radioactivity than the Three Mile Island accident four months earlier.
The Cimarron Fuel Fabrication Site was a nuclear fuel production facility located by the Cimarron River near Cimarron City, Oklahoma. It was operated by Kerr-McGee Corporation (KMC) from 1965 to 1975.

Ambrosia Lake is a uranium mining district in McKinley and Cibola counties in New Mexico north of Grants that was heavily mined for uranium starting in the 1950s. It is in an anticlinal dome.

Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is an orange volatile crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.
ConverDyn is a general partnership between American multinational firms General Atomics and Honeywell that provides uranium hexafluoride (UF6) conversion and related services to utilities operating nuclear power plants in North America, Europe, and Asia. The company is the sole marketing agent of UF6 produced at the Honeywell Uranium Hexafluoride Processing Facility in Metropolis, Illinois.

Nuclear labor issues exist within the international nuclear power industry and the nuclear weapons production sector worldwide, impacting upon the lives and health of laborers, itinerant workers and their families.
Depleted uranium hexafluoride (DUHF; also referred to as depleted uranium tails, depleted uranium tailings or DUF6) is a byproduct of the processing of uranium hexafluoride into enriched uranium. It is one of the chemical forms of depleted uranium (up to 73-75%), along with depleted triuranium octoxide (up to 25%) and depleted uranium metal (up to 2%). DUHF is 1.7 times less radioactive than uranium hexafluoride and natural uranium.












