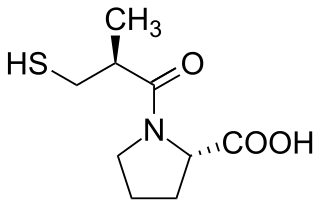
Angiotensin-converting-enzyme inhibitors are a class of medication used primarily for the treatment of high blood pressure and heart failure. This class of medicine works by causing relaxation of blood vessels as well as a decrease in blood volume, which leads to lower blood pressure and decreased oxygen demand from the heart.

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.

Cardiac muscle is one of three types of vertebrate muscle tissues, with the other two being skeletal muscle and smooth muscle. It is an involuntary, striated muscle that constitutes the main tissue of the wall of the heart. The cardiac muscle (myocardium) forms a thick middle layer between the outer layer of the heart wall and the inner layer, with blood supplied via the coronary circulation. It is composed of individual cardiac muscle cells joined by intercalated discs, and encased by collagen fibers and other substances that form the extracellular matrix.
Atrial natriuretic peptide (ANP) or atrial natriuretic factor (ANF) is a natriuretic peptide hormone secreted from the cardiac atria that in humans is encoded by the NPPA gene. Natriuretic peptides are a family of hormone/paracrine factors that are structurally related. The main function of ANP is causing a reduction in expanded extracellular fluid (ECF) volume by increasing renal sodium excretion. ANP is synthesized and secreted by cardiac muscle cells in the walls of the atria in the heart. These cells contain volume receptors which respond to increased stretching of the atrial wall due to increased atrial blood volume.

Vasodilation, also known as vasorelaxation, is the widening of blood vessels. It results from relaxation of smooth muscle cells within the vessel walls, in particular in the large veins, large arteries, and smaller arterioles. The process is the opposite of vasoconstriction, which is the narrowing of blood vessels.

In haemodynamics, the body must respond to physical activities, external temperature, and other factors by homeostatically adjusting its blood flow to deliver nutrients such as oxygen and glucose to stressed tissues and allow them to function. Haemodynamic response (HR) allows the rapid delivery of blood to active neuronal tissues. The brain consumes large amounts of energy but does not have a reservoir of stored energy substrates. Since higher processes in the brain occur almost constantly, cerebral blood flow is essential for the maintenance of neurons, astrocytes, and other cells of the brain. This coupling between neuronal activity and blood flow is also referred to as neurovascular coupling.
Relaxin is a protein hormone of about 6000 Da, first described in 1926 by Frederick Hisaw.

Carvedilol, sold under the brand name Coreg among others, is a medication used to treat high blood pressure, congestive heart failure (CHF), and left ventricular dysfunction in people who are otherwise stable. For high blood pressure, it is generally a second-line treatment. It is taken by mouth.

The adenosine A1 receptor (A1AR) is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand.
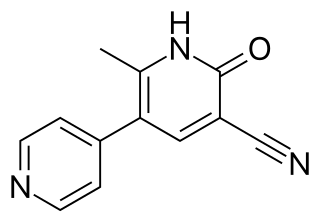
Milrinone, sold under the brand name Primacor, is a pulmonary vasodilator used in patients who have heart failure. It is a phosphodiesterase 3 inhibitor that works to increase the heart's contractility and decrease pulmonary vascular resistance. Milrinone also works to vasodilate which helps alleviate increased pressures (afterload) on the heart, thus improving its pumping action. While it has been used in people with heart failure for many years, studies suggest that milrinone may exhibit some negative side effects that have caused some debate about its use clinically.

Nebivolol is a beta blocker used to treat high blood pressure and heart failure. As with other β-blockers, it is generally a less preferred treatment for high blood pressure. It may be used by itself or with other blood pressure medication. It is taken by mouth.
Urocortin 2 (Ucn2) is an endogenous peptide in the corticotrophin-releasing factor (CRF) family.

Takotsubo cardiomyopathy or takotsubo syndrome (TTS), also known as stress cardiomyopathy, is a type of non-ischemic cardiomyopathy in which there is a sudden temporary weakening of the muscular portion of the heart. It usually appears after a significant stressor, either physical or emotional; when caused by the latter, the condition is sometimes called broken heart syndrome. Examples of physical stressors that can cause TTS are sepsis, shock, subarachnoid hemorrhage, and pheochromocytoma. Emotional stressors include bereavement, divorce, or the loss of a job. Reviews suggest that of patients diagnosed with the condition, about 70–80% recently experienced a major stressor, including 41–50% with a physical stressor and 26–30% with an emotional stressor. TTS can also appear in patients who have not experienced major stressors.
Biological functions of nitric oxide are roles that nitric oxide plays within biology.

Riociguat, sold under the brand name Adempas, is a medication by Bayer that is a stimulator of soluble guanylate cyclase (sGC). It is used to treat two forms of pulmonary hypertension (PH): chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension (PAH). Riociguat constitutes the first drug of the class of sGC stimulators. The drug has a half-life of 12 hours and will decrease dyspnea associated with pulmonary arterial hypertension.

Coronary perfusion pressure (CPP) refers to the pressure gradient that drives coronary blood pressure. The heart's function is to perfuse blood to the body; however, the heart's own myocardium must, itself, be supplied for its own muscle function. The heart is supplied by coronary vessels, and therefore CPP is the blood pressure within those vessels. If pressures are too low in the coronary vasculature, then the myocardium risks ischemia with subsequent myocardial infarction or cardiogenic shock.

Omecamtiv mecarbil (INN), previously referred to as CK-1827452, is a cardiac-specific myosin activator. It is being studied for a potential role in the treatment of left ventricular systolic heart failure.
CXL 1020 is an experimental drug that is being investigated as a treatment for acute decompensated heart failure. CXL 1020 functions as a nitroxyl donor; nitroxyl is the reduced, protonated version of nitric oxide. Nitroxyl is capable of enhancing left ventricular contractility without increasing heart rate by modifying normal Ca2+ cycling through the sarcoplasmic reticulum as well as increasing the sensitivity of cardiac myofilaments to Ca2+.
The β3 adrenergic receptor agonist or β3-adrenoceptor agonist, also known as β3-AR agonist, are a class of medicine that bind selectively to β3-adrenergic receptors.

Brain natriuretic peptide 32 (BNP), also known as B-type natriuretic peptide, is a hormone secreted by cardiomyocytes in the heart ventricles in response to stretching caused by increased ventricular blood volume. Along with NT-proBNP, BNP is one of two natriuretic peptides.














