
The thymus is a specialized primary lymphoid organ of the immune system. Within the thymus, thymus cell lymphocytes or T cells mature. T cells are critical to the adaptive immune system, where the body adapts to specific foreign invaders. The thymus is located in the upper front part of the chest, in the anterior superior mediastinum, behind the sternum, and in front of the heart. It is made up of two lobes, each consisting of a central medulla and an outer cortex, surrounded by a capsule.

Severe combined immunodeficiency (SCID), also known as Swiss-type agammaglobulinemia, is a rare genetic disorder characterized by the disturbed development of functional T cells and B cells caused by numerous genetic mutations that result in differing clinical presentations. SCID involves defective antibody response due to either direct involvement with B lymphocytes or through improper B lymphocyte activation due to non-functional T-helper cells. Consequently, both "arms" of the adaptive immune system are impaired due to a defect in one of several possible genes. SCID is the most severe form of primary immunodeficiencies, and there are now at least nine different known genes in which mutations lead to a form of SCID. It is also known as the bubble boy disease and bubble baby disease because its victims are extremely vulnerable to infectious diseases and some of them, such as David Vetter, have become famous for living in a sterile environment. SCID is the result of an immune system so highly compromised that it is considered almost absent.
Mouse mammary tumor virus (MMTV) is a milk-transmitted retrovirus like the HTL viruses, HI viruses, and BLV. It belongs to the genus Betaretrovirus. MMTV was formerly known as Bittner virus, and previously the "milk factor", referring to the extra-chromosomal vertical transmission of murine breast cancer by adoptive nursing, demonstrated in 1936, by John Joseph Bittner while working at the Jackson Laboratory in Bar Harbor, Maine. Bittner established the theory that a cancerous agent, or "milk factor", could be transmitted by cancerous mothers to young mice from a virus in their mother's milk. The majority of mammary tumors in mice are caused by mouse mammary tumor virus.

Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the very first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm.
Lymphoproliferative disorders (LPDs) refer to a specific class of diagnoses, comprising a group of several conditions, in which lymphocytes are produced in excessive quantities. These disorders primarily present in patients who have a compromised immune system. Due to this factor, there are instances of these conditions being equated with "immunoproliferative disorders"; although, in terms of nomenclature, lymphoproliferative disorders are a subclass of immunoproliferative disorders—along with hypergammaglobulinemia and paraproteinemias.

ZAP70 deficiency, or ZAP70 deficient SCID, is a rare autosomal recessive form of severe combined immunodeficiency (SCID) resulting in a lack of CD8+ T cells. People with this disease lack the capability to fight infections, and it is fatal if untreated.

X-linked severe combined immunodeficiency (X-SCID) is an immunodeficiency disorder in which the body produces very few T cells and NK cells.
Primary immunodeficiencies are disorders in which part of the body's immune system is missing or does not function normally. To be considered a primary immunodeficiency (PID), the immune deficiency must be inborn, not caused by secondary factors such as other disease, drug treatment, or environmental exposure to toxins. Most primary immunodeficiencies are genetic disorders; the majority are diagnosed in children under the age of one, although milder forms may not be recognized until adulthood. While there are over 430 recognized inborn errors of immunity (IEIs) as of 2019, the vast majority of which are PIDs, most are very rare. About 1 in 500 people in the United States are born with a primary immunodeficiency. Immune deficiencies can result in persistent or recurring infections, auto-inflammatory disorders, tumors, and disorders of various organs. There are currently limited treatments available for these conditions; most are specific to a particular type of PID. Research is currently evaluating the use of stem cell transplants (HSCT) and experimental gene therapies as avenues for treatment in limited subsets of PIDs.

John Edgar Dick is Canada Research Chair in Stem Cell Biology, Senior Scientist at the Princess Margaret Cancer Centre, University Health Network and Professor in the Department of Molecular Genetics at the University of Toronto in Canada. Dick is credited with first identifying cancer stem cells in certain types of human leukemia. His revolutionary findings highlighted the importance of understanding that not all cancer cells are the same and thus spawned a new direction in cancer research. Dick is also known for his demonstration of a blood stem cell's ability to replenish the blood system of a mouse, his development of a technique to enable an immune-deficient mouse to carry and produce human blood, and his creation of the world's first mouse with human leukemia.

Tyrosine-protein kinase JAK3 is a tyrosine kinase enzyme that in humans is encoded by the JAK3 gene.
The severe combined immunodeficiency (SCID) is a severe immunodeficiency genetic disorder that is characterized by the complete inability of the adaptive immune system to mount, coordinate, and sustain an appropriate immune response, usually due to absent or atypical T and B lymphocytes. In humans, SCID is colloquially known as "bubble boy" disease, as victims may require complete clinical isolation to prevent lethal infection from environmental microbes.
A NOG (NOD/Shi-scid/IL-2Rγnull) mouse is a new generation of severely immunodeficient mouse, developed by Central Institute for Experimental Animals (CIEA) in 2000. The NOG mouse accepts heterologous cells much more easily compared with any other type of immunodeficient rodent models, such as nude mouse and NOD/scid mouse. Thus, the mouse can be the best model as a highly efficient recipient of human cells to engraft, proliferate and differentiate. This unique feature offers a great opportunity for enhancing therapy researches of cancer, leukemia, visceral diseases, AIDS, and other human diseases. It also provides applications for cancer, infection, regeneration, and hematology researches.
A humanized mouse is a mouse carrying functioning human genes, cells, tissues, and/or organs. Humanized mice are commonly used as small animal models in biological and medical research for human therapeutics.
The NSG mouse is a brand of immunodeficient laboratory mice, developed and marketed by Jackson Laboratory, which carries the strain NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ. NSG branded mice are among the most immunodeficient described to date. NSG branded mice lack mature T cells, B cells, and natural killer (NK) cells. NSG branded mice are also deficient in multiple cytokine signaling pathways, and they have many defects in innate immunity. The compound immunodeficiencies in NSG branded mice permit the engraftment of a wide range of primary human cells, and enable sophisticated modeling of many areas of human biology and disease. NSG branded mice were developed in the laboratory of Dr. Leonard Shultz at Jackson Laboratory, which owns the NSG trade mark.

Reticular dysgenesis (RD) is a rare, inherited autosomal recessive disease that results in immunodeficiency. Individuals with RD have mutations in both copies of the AK2 gene. Mutations in this gene lead to absence of AK2 protein. AK2 protein allows hematopoietic stem cells to differentiate and proliferate. Hematopoietic stem cells give rise to blood cells.
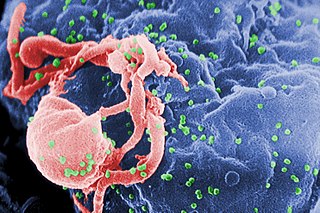
HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
Patient derived xenografts (PDX) are models of cancer where the tissue or cells from a patient's tumor are implanted into an immunodeficient or humanized mouse. It is a form of xenotransplantation. PDX models are used to create an environment that allows for the continued growth of cancer after its removal from a patient. In this way, tumor growth can be monitored in the laboratory, including in response to potential therapeutic options. Cohorts of PDX models can be used to determine the therapeutic efficiency of a therapy against particular types of cancer, or a PDX model from a specific patient can be tested against a range of therapies in a 'personalized oncology' approach.
Autologous CD34+ enriched cell fraction that contains CD34+ cells transduced with retroviral vector that encodes for the human ADA cDNA sequence, sold under the brand name Strimvelis, is a medication used to treat severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID).
T-cell depletion (TCD) is the process of T cell removal or reduction, which alters the immune system and its responses. Depletion can occur naturally or be induced for treatment purposes. TCD can reduce the risk of graft-versus-host disease (GVHD), which is a common issue in transplants. The idea that TCD of the allograft can eliminate GVHD was first introduced in 1958. In humans the first TCD was performed in severe combined immunodeficiency patients.

The laboratory mouse has been instrumental in investigating the genetics of human disease, including cancer, for over 110 years. The laboratory mouse has physiology and genetic characteristics very similar to humans providing powerful models for investigation of the genetic characteristics of disease.