 | |
| Names | |
|---|---|
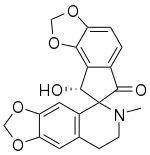
| IUPAC name 8'-Hydroxy-6-methylspiro[7,8-dihydro-[1,3]dioxolo[4,5-g]isoquinoline-5,7'-8H-cyclopenta[g][1,3]benzodioxole]-6'-one | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C20H17NO6 | |
| Molar mass | 367.357 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sibiricine is a bioactive isoquinoline alkaloid isolated from Corydalis crispa (Fumariaceae), which is a Bhutanese medicinal plant from the Himalayas. [3] [4]
Using high resolution mass spectrometry, the molecular formula of sibiricine is determined to be C20H17NO6. [5] The IUPAC name for sibiricine is 8'-hydroxy-6-methylspiro[7,8-dihydro-[1,3]dioxolo[4,5-g]isoquinoline-5,7'-8H-cyclopenta[g][1,3]benzodioxole]-6'-one. [6] The proton nuclear magnetic resonance (PMR) spectrum of sibiricine at 100 MHz shows that sibiricine is structurally related to ochrobirine and ochotensine. [3] [5] With the exception of sibiricine, 8 other alkaloids are extracted by investigating Corydalis crispa. These isoquinoline alkaloids are protopine, 13-oxoprotopine, 13-oxocryptopine, stylopine, coreximine, rheagenine, ochrobirine, and bicuculline. [3]