
The Epstein–Barr virus (EBV), formally called Human gammaherpesvirus 4, is one of the nine known human herpesvirus types in the herpes family, and is one of the most common viruses in humans. EBV is a double-stranded DNA virus.

A poliovirus, the causative agent of polio, is a serotype of the species Enterovirus C, in the family of Picornaviridae. There are three poliovirus serotypes: types 1, 2, and 3.

Rhabdoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates, invertebrates, plants, fungi and protozoans serve as natural hosts. Diseases associated with member viruses include rabies encephalitis caused by the rabies virus, and flu-like symptoms in humans caused by vesiculoviruses. The name is derived from Ancient Greek rhabdos, meaning rod, referring to the shape of the viral particles. The family has 40 genera, most assigned to three subfamilies.

Picornaviruses are a group of related nonenveloped RNA viruses which infect vertebrates including fish, mammals, and birds. They are viruses that represent a large family of small, positive-sense, single-stranded RNA viruses with a 30 nm icosahedral capsid. The viruses in this family can cause a range of diseases including the common cold, poliomyelitis, meningitis, hepatitis, and paralysis.
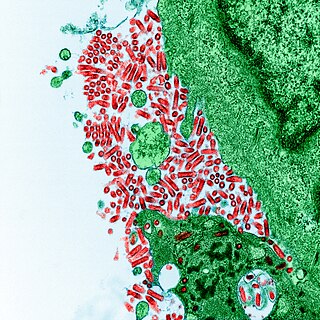
Rabies virus, scientific name Rabies lyssavirus, is a neurotropic virus that causes rabies in humans and animals. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva. Rabies lyssavirus, like many rhabdoviruses, has an extremely wide host range. In the wild it has been found infecting many mammalian species, while in the laboratory it has been found that birds can be infected, as well as cell cultures from mammals, birds, reptiles and insects. Rabies is reported in more than 150 countries on all continents, with the exclusion of Antarctica. The main burden of disease is reported in Asia and Africa, but some cases have been reported also in Europe in the past 10 years, especially in returning travellers.

Birnaviridae is a family of double-stranded RNA viruses. Salmonid fish, birds and insects serve as natural hosts. There are currently 11 species in this family, divided among seven genera. Diseases associated with this family include infectious pancreatic necrosis in salmonid fish, which causes significant losses to the aquaculture industry, with chronic infection in adult salmonid fish and acute viral disease in young salmonid fish.

An arenavirus is a bi- or trisegmented ambisense RNA virus that is a member of the family Arenaviridae. These viruses infect rodents and occasionally humans. A class of novel, highly divergent arenaviruses, properly known as reptarenaviruses, have also been discovered which infect snakes to produce inclusion body disease. At least eight arenaviruses are known to cause human disease. The diseases derived from arenaviruses range in severity. Aseptic meningitis, a severe human disease that causes inflammation covering the brain and spinal cord, can arise from the lymphocytic choriomeningitis virus. Hemorrhagic fever syndromes, including Lassa fever, are derived from infections such as Guanarito virus, Junin virus, Lassa virus, Lujo virus, Machupo virus, Sabia virus, or Whitewater Arroyo virus. Because of the epidemiological association with rodents, some arenaviruses and bunyaviruses are designated as roboviruses.
Viral pathogenesis is the study of the process and mechanisms by which viruses cause diseases in their target hosts, often at the cellular or molecular level. It is a specialized field of study in virology.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.
Simian foamy virus (SFV) is a species of the genus Spumavirus that belongs to the family of Retroviridae. It has been identified in a wide variety of primates, including prosimians, New World and Old World monkeys, as well as apes, and each species has been shown to harbor a unique (species-specific) strain of SFV, including African green monkeys, baboons, macaques, and chimpanzees. As it is related to the more well-known retrovirus human immunodeficiency virus (HIV), its discovery in primates has led to some speculation that HIV may have been spread to the human species in Africa through contact with blood from apes, monkeys, and other primates, most likely through bushmeat-hunting practices.

Murine coronavirus (M-CoV) is a virus in the genus Betacoronavirus that infects mice. Belonging to the subgenus Embecovirus, murine coronavirus strains are enterotropic or polytropic. Enterotropic strains include mouse hepatitis virus (MHV) strains D, Y, RI, and DVIM, whereas polytropic strains, such as JHM and A59, primarily cause hepatitis, enteritis, and encephalitis. Murine coronavirus is an important pathogen in the laboratory mouse and the laboratory rat. It is the most studied coronavirus in animals other than humans, and has been used as an animal disease model for many virological and clinical studies.
Seoul orthohantavirus (SEOV) is a member of the Orthohantavirus family of rodent-borne viruses and is one of the 4 hantaviruses that are known to be able to cause Hantavirus hemorrhagic fever with renal syndrome (HFRS). It is an Old World hantavirus; a negative sense, single-stranded, tri-segmented RNA virus.

Viral hemorrhagic septicemia (VHS) is a deadly infectious fish disease caused by Viral hemorrhagic septicemia virus. It afflicts over 50 species of freshwater and marine fish in several parts of the Northern Hemisphere. Different strains of the virus occur in different regions, and affect different species. There are no signs that the disease affects human health. VHS is also known as Egtved disease, and the virus as Egtved virus.
Visna-maedi virus from the genus Lentivirus and subfamily Orthoretrovirinae, is a retrovirus that causes encephalitis and chronic pneumonitis in sheep. It is known as visna when found in the brain, and maedi when infecting the lungs. Lifelong, persistent infections in sheep occur in the lungs, lymph nodes, spleen, joints, central nervous system, and mammary glands; The condition is sometimes known as ovine progressive pneumonia (OPP), particularly in the United States, or Montana sheep disease. White blood cells of the monocyte/macrophage lineage are the main target of the virus.
Infectious hematopoietic necrosis virus (IHNV), is a negative-sense single-stranded, bullet-shaped RNA virus that is a member of the Rhabdoviridae family, and from the genus Novirhabdovirus. It causes the disease known as infectious hematopoietic necrosis in salmonid fish such as trout and salmon. The disease may be referred to by a number of other names such as Chinook salmon disease, Coleman disease, Columbia River sockeye disease, Cultus Lake virus disease, Oregon sockeye disease, Sacramento River Chinook disease and sockeye salmon viral disease. IHNV is commonly found in the Pacific Coast of Canada and the United States, and has also been found in Europe and Japan. The first reported epidemics of IHNV occurred in the United States at the Washington and the Oregon fish hatcheries during the 1950s. IHNV is transmitted following shedding of the virus in the feces, urine, sexual fluids, and external mucus and by direct contact or close contact with surrounding water. The virus gains entry into fish at the base of the fins.
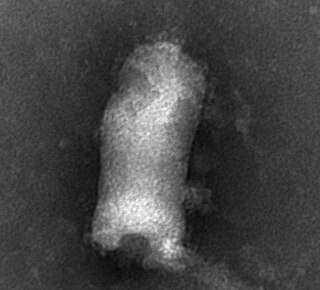
Novirhabdovirus is a genus of the family Rhabdoviridae containing viruses known to infect aquatic hosts. They can be transmitted from fish to fish or by waterborne virus, as well as through contaminated eggs. Replication and thermal inactivation temperatures are generally lower than for other rhabdoviruses, given the cold-blooded nature of their hosts. Hosts include a large and growing range of marine and freshwater fish.
Avian sarcoma leukosis virus (ASLV) is an endogenous retrovirus that infects and can lead to cancer in chickens; experimentally it can infect other species of birds and mammals. ASLV replicates in chicken embryo fibroblasts, the cells that contribute to the formation of connective tissues. Different forms of the disease exist, including lymphoblastic, erythroblastic, and osteopetrotic.
Feline coronavirus (FCoV) is a positive-stranded RNA virus that infects cats worldwide. It is a coronavirus of the species Alphacoronavirus 1 which includes canine coronavirus (CCoV) and porcine transmissible gastroenteritis coronavirus (TGEV). It has two different forms: feline enteric coronavirus (FECV) that infects the intestines and feline infectious peritonitis virus (FIPV) that causes the disease feline infectious peritonitis (FIP).
Equine foamy virus (EFV), also called foamy virus (FV), is virus in the genus Equispumavirus. It shares similarities, with respect to replication, with lentiviruses. EFV, along with other FVs are from the family Retroviridae and subfamily Spumaretrovirinae. Spumarivuses, such as EFV, are complicated retroviruses that have been characterized in many animals including nonhuman primates, cattle, cats. Additionally, these viruses have been identified in animals that most often carry lentiviruses.

The woolly monkey hepatitis B virus (WMHBV) is a viral species of the Orthohepadnavirus genus of the Hepadnaviridae family. Its natural host is the woolly monkey (Lagothrix), an inhabitant of South America categorized as a New World primate. WMHBV, like other hepatitis viruses, infects the hepatocytes, or liver cells, of its host organism. It can cause hepatitis, liver necrosis, cirrhosis, and hepatocellular carcinoma. Because nearly all species of Lagothrix are threatened or endangered, researching and developing a vaccine and/or treatment for WMHBV is important for the protection of the whole woolly monkey genus.











