
Cefuroxime, sold under the brand name Zinacef among others, is a second-generation cephalosporin antibiotic used to treat and prevent a number of bacterial infections. These include pneumonia, meningitis, otitis media, sepsis, urinary tract infections, and Lyme disease. It is used by mouth or by injection into a vein or muscle.

Enoxaparin sodium, sold under the brand name Lovenox among others, is an anticoagulant medication. It is used to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE) including during pregnancy and following certain types of surgery. It is also used in those with acute coronary syndrome (ACS) and heart attacks. It is given by injection just under the skin or into a vein. It is also used during hemodialysis.
Pegfilgrastim, sold under the brand name Neulasta among others, is a PEGylated form of the recombinant human granulocyte colony-stimulating factor (GCSF) analog filgrastim. It serves to stimulate the production of white blood cells (neutrophils). Pegfilgrastim was developed by Amgen.

Insulin aspart, sold under the brand name NovoLog and Fiasp, among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is generally used by injection under the skin but may also be used by injection into a vein. Maximum effect occurs after about 1–3 hours and lasts for 3–5 hours. Generally a longer-acting insulin like insulin NPH is also needed.

Isavuconazonium sulfate, sold under the brand name Cresemba, is a systemic antifungal medication of the triazole class which is used to treat invasive aspergillosis and mucormycosis.
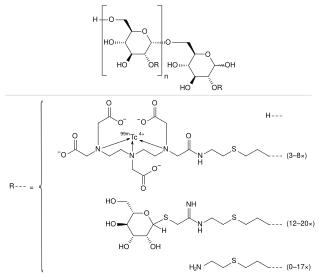
Technetium (99mTc) tilmanocept, trade name Lymphoseek, is a radiopharmaceutical diagnostic imaging agent used to locate lymph nodes which may be draining from tumors, and assist doctors in locating lymph nodes for removal during surgery.

Cabotegravir, sold under the brand name Vocabria among others, is a antiretroviral medication used for the treatment of HIV/AIDS. It is available in the form of tablets and as an intramuscular injection, as well as in an injectable combination with rilpivirine under the brand name Cabenuva.

Empagliflozin/linagliptin, sold under the brand name Glyxambi, is a fixed-dose combination anti-diabetic medication used to treat type 2 diabetes. It is a combination of empagliflozin and linagliptin. It is taken by mouth.
Sebelipase alfa, sold under the brand name Kanuma, is a recombinant form of the enzyme lysosomal acid lipase (LAL) that is used as a medication for the treatment of lysosomal acid lipase deficiency (LAL-D). It is administered via intraveneous infusion. It was approved for medical use in the European Union and in the United States in 2015.

Siponimod, sold under the brand name Mayzent, is a selective sphingosine-1-phosphate receptor modulator for oral use that is used for multiple sclerosis (MS). It is intended for once-daily oral administration.

Semaglutide, sold under the brand names Ozempic, Wegovy and Rybelsus, is an antidiabetic medication used for the treatment of type 2 diabetes and an anti-obesity medication used for long-term weight management, developed by Novo Nordisk in 2012. It is a peptide similar to the hormone glucagon-like peptide-1 (GLP-1), modified with a side chain. It can be administered by subcutaneous injection or taken orally.
Insulin degludec/liraglutide, sold under the brand name Xultophy, is a fixed-dose combination medication for the treatment of adults with type 2 diabetes to improve glycemic control in combination with diet and exercise. It contains insulin degludec and liraglutide. It is administered by subcutaneous injection.
Burosumab, sold under the brand name Crysvita, is a human monoclonal antibody medication approved 2018 for the treatment of X-linked hypophosphatemia and tumor-induced osteomalacia.
Hepatitis A and typhoid vaccine is a combination vaccine to protect against the infectious diseases hepatitis A and typhoid. It is a combination of inactivated Hepatitis A virus and Vi polysaccharide of Salmonella typhi bacteria. Branded formulations include Hepatyrix from GlaxoSmithKline, and ViVaxim and ViATIM from Sanofi Pasteur.
Empagliflozin/metformin, sold under the brand name Synjardy among others, is a fixed-dose combination anti-diabetic medication used to treat type 2 diabetes. It contains empagliflozin and metformin hydrochloride. It is taken by mouth.
Ferric derisomaltose (FDI), sold under the brand name Monoferric among others, is a medication for the treatment of iron deficiency anemia (IDA) in adults who have intolerance to oral iron or have had unsatisfactory response to oral iron or who have non-hemodialysis dependent chronic kidney disease (NDD-CKD). It was approved for use in the United States in January 2020. It is given intravenously.
Susoctocog alfa, sold under the brand name Obizur, is a medication used for the treatment of bleeding episodes in adults with acquired haemophilia, a bleeding disorder caused by the spontaneous development of antibodies that inactivate factor VIII.
Vonicog alfa, sold under the brand names Vonvendi and Veyvondi, is a medication used to control bleeding in adults with von Willebrand disease. It is a recombinant von Willebrand factor.
Efmoroctocog alfa, sold under the brand name Elocta among others, is a medication for the treatment and prophylaxis of bleeding in people with hemophilia A. Efmoroctocog alfa is a recombinant human coagulation factor VIII, Fc fusion protein (rFVIIIFc). It is produced by recombinant DNA technology in a human embryonic kidney (HEK) cell line.

Lenacapavir, sold under the brand name Sunlenca, is an antiretroviral medication used to treat HIV/AIDS. It is taken by mouth or by subcutaneous injection.










