
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salting), smoke (smoking), sugar (crystallization), etc. This allows for longer-lasting foods such as bacon, sweets or wines. With the advent of processed foods in the second half of the twentieth century, many additives have been introduced, of both natural and artificial origin. Food additives also include substances that may be introduced to food indirectly in the manufacturing process, through packaging, or during storage or transport.

Sodium chloride, commonly known as table salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather.

Sourdough or sourdough bread is a bread made by the fermentation of dough using wild lactobacillaceae and yeast. Lactic acid from fermentation imparts a sour taste and improves keeping qualities.

Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Salts and esters of adipic acid are known as adipates.

Carboxymethyl cellulose (CMC) or cellulose gum is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is often used as its sodium salt, sodium carboxymethyl cellulose. It used to be marketed under the name Tylose, a registered trademark of SE Tylose.
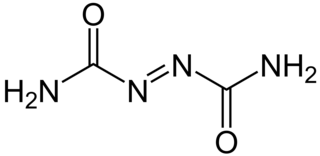
Azodicarbonamide, ADCA, ADA, or azo(bis)formamide, is a chemical compound with the molecular formula C2H4O2N4. It is a yellow to orange-red, odorless, crystalline powder. It is sometimes called a 'yoga mat' chemical because of its widespread use in foamed plastics. It was first described by John Bryden in 1959.
Phosphated distarch phosphate, is a type of chemically modified starch. It can be derived from wheat starch, tapioca starch, potato starch or many other botanical sources of starch. It is produced by replacing the hydrogen bonds between starch chains by stronger, covalent phosphate bonds that are more permanent. It is manufactured by treating starch with sodium tripolyphosphate (STPP) and sodium trimetaphosphate (STMP), or phosphoric chloride (POCl3). Phosphorylated cross-linked starches is a category of modified food starches within the U.S. Code of Federal Regulations. Starches treated with STMP and STPP must not exceed 0.4 percent phosphorus as residual phosphate.
Calcium propanoate or calcium propionate has the formula Ca(C2H5COO)2. It is the calcium salt of propanoic acid.

Caramel color or caramel coloring is a water-soluble food coloring. It is made by heat treatment of carbohydrates (sugars), in general in the presence of acids, alkalis, or salts, in a process called caramelization. It is more fully oxidized than caramel candy, and has an odor of burnt sugar and a somewhat bitter taste. Its color ranges from pale yellow to amber to dark brown.

Modified starch, also called starch derivatives, are prepared by physically, enzymatically, or chemically treating native starch to change its properties. Modified starches are used in practically all starch applications, such as in food products as a thickening agent, stabilizer or emulsifier; in pharmaceuticals as a disintegrant; or as binder in coated paper. They are also used in many other applications.
Food chemistry is the study of chemical processes and interactions of all biological and non-biological components of foods. The biological substances include such items as meat, poultry, lettuce, beer, milk as examples. It is similar to biochemistry in its main components such as carbohydrates, lipids, and protein, but it also includes areas such as water, vitamins, minerals, enzymes, food additives, flavors, and colors. This discipline also encompasses how products change under certain food processing techniques and ways either to enhance or to prevent them from happening. An example of enhancing a process would be to encourage fermentation of dairy products with microorganisms that convert lactose to lactic acid; an example of preventing a process would be stopping the browning on the surface of freshly cut apples using lemon juice or other acidulated water.
The sponge and dough method is a two-step bread making process: in the first step a sponge is made and allowed to ferment for a period of time, and in the second step the sponge is added to the final dough's ingredients, creating the total formula. In this usage, synonyms for sponge are yeast starter or yeast pre-ferment. In French baking the sponge and dough method is known as levain-levure. The method is reminiscent of the sourdough or levain methods; however, the sponge is made from all fresh ingredients prior to being used in the final dough.

Sodium lactate is the sodium salt of lactic acid, and has a mild saline taste. It is produced by fermentation of a sugar source, such as corn or beets, and then, by neutralizing the resulting lactic acid to create a compound having the formula NaC3H5O3.

A dough conditioner, flour treatment agent, improving agent or bread improver is any ingredient or chemical added to bread dough to strengthen its texture or otherwise improve it in some way. Dough conditioners may include enzymes, yeast nutrients, mineral salts, oxidants and reductants, bleaching agents and emulsifiers. They are food additives combined with flour to improve baking functionality. Flour treatment agents are used to increase the speed of dough rising and to improve the strength and workability of the dough.
Synthetic magnesium silicates are white, odorless, finely divided powders formed by the precipitation reaction of water-soluble sodium silicate and a water-soluble magnesium salt such as magnesium chloride, magnesium nitrate or magnesium sulfate. The composition of the precipitate depends on the ratio of the components in the reaction medium, the addition of the correcting substances, and the way in which they are precipitated.
Lactylates are organic compounds that are FDA approved for use as food additives and cosmetic ingredients, e.g. as food-grade emulsifiers. These additives are non-toxic, biodegradable, and typically manufactured using biorenewable feedstocks. Owing to their safety and versatile functionality, lactylates are used in a wide variety of food and non-food applications. In the United States, the Food Chemicals Codex specifies the labeling requirements for food ingredients including lactylates. In the European Union, lactylates must be labelled in accordance with the requirements of the applicable EU regulation. Lactylates may be labelled as calcium stearoyl lactylate (CSL), sodium stearoyl lactylate (SSL), or lactylic esters of fatty acids (LEFA).
Calcium stearoyl-2-lactylate or E482 is a versatile, FDA approved food additive. It is one type of a commercially available lactylate. CSL is non-toxic, biodegradable, and typically manufactured using biorenewable feedstocks. Because CSL is a safe and highly effective food additive, it is used in a wide variety of products from baked goods and desserts to packaging.

Sour cream is a dairy product obtained by fermenting regular cream with certain kinds of lactic acid bacteria. The bacterial culture, which is introduced either deliberately or naturally, sours and thickens the cream. Its name comes from the production of lactic acid by bacterial fermentation, which is called souring. Crème fraîche is one type of sour cream with a high fat content and less sour taste.
The Food Chemicals Codex (FCC) is a collection of internationally recognized standards for the purity and identity of food ingredients.











