
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation with their smell.
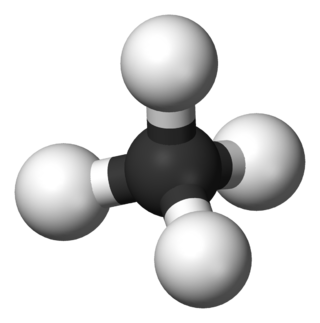
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring.

In chemistry, aromaticity means the molecule has cyclic (ring-shaped) structures with pi bonds in resonance. Aromatic rings give increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term aromaticity with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.

In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.

Sphingomonas was defined in 1990 as a group of Gram-negative, rod-shaped, chemoheterotrophic, strictly aerobic bacteria. They possess ubiquinone 10 as their major respiratory quinone, contain glycosphingolipids (GSLs), specifically ceramide, instead of lipopolysaccharide (LPS) in their cell envelopes, and typically produce yellow-pigmented colonies. The GSL serves to protect the bacteria from antibacterial substances. Unlike most Gram-negative bacteria, Sphingomonas carries endotoxins and has a hydrophobic surface characterized by the short nature of the GSL's carbohydrate portion.

Sphingomonadaceae are a gram-negative bacterial family of the Alphaproteobacteria. An important feature is the presence of sphingolipids in the outer membrane of the cell wall. The cells are ovoid or rod-shaped. Others are also pleomorphic, i.e. the cells change the shape over time. Some species from Sphingomonadaceae family are dominant components of biofilms.

An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field.
Novosphingobium is a genus of Gram-negative bacteria that includes N. taihuense, which can degrade aromatic compounds such as phenol, aniline, nitrobenzene and phenanthrene. The species N. aromativorans, which was first found in Ulsan Bay, similarly degrades aromatic molecules of two to five rings.
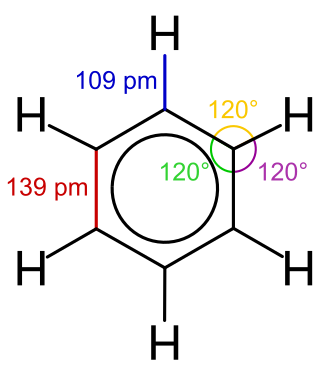
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.

Sphingobium chlorophenolicum is a species of bacteria.
The enzyme pyrethroid hydrolase (EC 3.1.1.88, pyrethroid-hydrolyzing carboxylesterase, pyrethroid-hydrolysing esterase, pyrethroid-hydrolyzing esterase, pyrethroid-selective esterase, pyrethroid-cleaving enzyme, permethrinase, PytH, EstP; systematic name pyrethroid-ester hydrolase) catalyses the reaction
Sphingomonas yanoikuyae is a short rod-shaped, strictly aerobic, Gram-negative, non-motile, non-spore-forming, chemoheterotrophic species of bacteria that is yellow or off-white in color. Its type strain is JCM 7371. It is notable for degrading a variety of aromatic compounds including biphenyl, naphthalene, phenanthrene, toluene, m-, and p-xylene. S. yanoikuyae was discovered by Brian Goodman on the southern coast of Papua New Guinea. However, Sphingomonas have a wide distribution across freshwater, seawater, and terrestrial habitats. This is due to the bacteria's ability to grow and survive under low-nutrient conditions as it can utilize a broad range of organic compounds.
Sphingomonas stygia is a species of bacteria. It is an aromatic compound-degrading bacteria, it is gram-negative, non-spore-forming, non-motile and rod-shaped. It is found in deep-terrestrial-subsurface sediments.
Sphingobium japonicum is a hexachlorocyclohexane-degrading bacteria with type strain MTCC 6362T. Its genome has been sequenced.
Sphingobium indicum is a hexachlorocyclohexane-degrading bacteria with type strain MTCC 6364T. Its genome has been sequenced.
Sphingobium francense is a hexachlorocyclohexane-degrading bacteria with type strain MTCC 6363T.
In organic chemistry, spherical aromaticity is formally used to describe an unusually stable nature of some spherical compounds such as fullerenes, polyhedral boranes.
Novosphingobium aromaticivorans is a species of bacteria. It is an aromatic compound-degrading bacteria, it is gram-negative, non-spore-forming, non-motile and rod-shaped. It is found in deep-terrestrial-subsurface sediments.










