
The central nervous system (CNS) is the part of the nervous system consisting of the brain and spinal cord, the retina and optic nerve, and the olfactory nerve and epithelia. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all parts of the bodies of bilaterally symmetric and triploblastic animals—that is, all multicellular animals except sponges and diploblasts. It is a structure composed of nervous tissue positioned along the rostral to caudal axis of the body and may have an enlarged section at the rostral end which is a brain. Only arthropods, cephalopods and vertebrates have a true brain, though precursor structures exist in onychophorans, gastropods and lancelets.
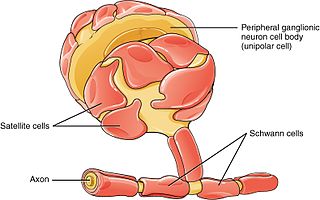
Schwann cells or neurolemmocytes are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcript that are again synthesized in a tissue-specific manner.
Myelitis is inflammation of the spinal cord which can disrupt the normal responses from the brain to the rest of the body, and from the rest of the body to the brain. Inflammation in the spinal cord can cause the myelin and axon to be damaged resulting in symptoms such as paralysis and sensory loss. Myelitis is classified to several categories depending on the area or the cause of the lesion; however, any inflammatory attack on the spinal cord is often referred to as transverse myelitis.
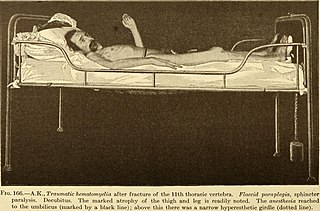
Paraplegia, or paraparesis, is an impairment in motor or sensory function of the lower extremities. The word comes from Ionic Greek (παραπληγίη) "half-stricken". It is usually caused by spinal cord injury or a congenital condition that affects the neural (brain) elements of the spinal canal. The area of the spinal canal that is affected in paraplegia is either the thoracic, lumbar, or sacral regions. If four limbs are affected by paralysis, tetraplegia or quadriplegia is the correct term. If only one limb is affected, the correct term is monoplegia. Spastic paraplegia is a form of paraplegia defined by spasticity of the affected muscles, rather than flaccid paralysis.

Functional electrical stimulation (FES) is a technique that uses low-energy electrical pulses to artificially generate body movements in individuals who have been paralyzed due to injury to the central nervous system. More specifically, FES can be used to generate muscle contraction in otherwise paralyzed limbs to produce functions such as grasping, walking, bladder voiding and standing. This technology was originally used to develop neuroprostheses that were implemented to permanently substitute impaired functions in individuals with spinal cord injury (SCI), head injury, stroke and other neurological disorders. In other words, a person would use the device each time he or she wanted to generate a desired function. FES is sometimes also referred to as neuromuscular electrical stimulation (NMES).

Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.
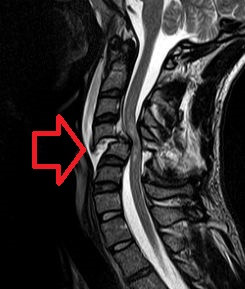
A spinal cord injury (SCI) is damage to the spinal cord that causes temporary or permanent changes in its function. It is a destructive neurological and pathological state that causes major motor, sensory and autonomic dysfunctions.
Neural engineering is a discipline within biomedical engineering that uses engineering techniques to understand, repair, replace, or enhance neural systems. Neural engineers are uniquely qualified to solve design problems at the interface of living neural tissue and non-living constructs.

Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells, they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.
Stem-cell therapy uses stem cells to treat or prevent a disease or condition. As of 2016, the only established therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone marrow transplantation, but the cells can also be derived from umbilical cord blood. Research is underway to develop various sources for stem cells as well as to apply stem-cell treatments for neurodegenerative diseases and conditions such as diabetes and heart disease.
Neuroepithelial cells, or neuroectodermal cells, form the wall of the closed neural tube in early embryonic development. The neuroepithelial cells span the thickness of the tube's wall, connecting with the pial surface and with the ventricular or lumenal surface. They are joined at the lumen of the tube by junctional complexes, where they form a pseudostratified layer of epithelium called neuroepithelium.
Neural stem cells (NSCs) are self-renewing, multipotent cells that firstly generate the radial glial progenitor cells that generate the neurons and glia of the nervous system of all animals during embryonic development. Some neural progenitor stem cells persist in highly restricted regions in the adult vertebrate brain and continue to produce neurons throughout life. Differences in the size of the central nervous system are among the most important distinctions between the species and thus mutations in the genes that regulate the size of the neural stem cell compartment are among the most important drivers of vertebrate evolution.
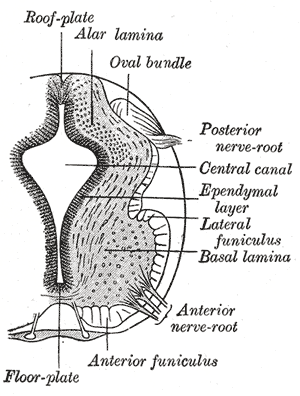
The floor plate is a structure integral to the developing nervous system of vertebrate organisms. Located on the ventral midline of the embryonic neural tube, the floor plate is a specialized glial structure that spans the anteroposterior axis from the midbrain to the tail regions. It has been shown that the floor plate is conserved among vertebrates, such as zebrafish and mice, with homologous structures in invertebrates such as the fruit fly Drosophila and the nematode C. elegans. Functionally, the structure serves as an organizer to ventralize tissues in the embryo as well as to guide neuronal positioning and differentiation along the dorsoventral axis of the neural tube.
Neuroregeneration involves the regrowth or repair of nervous tissues, cells or cell products. Neuroregenerative mechanisms may include generation of new neurons, glia, axons, myelin, or synapses. Neuroregeneration differs between the peripheral nervous system (PNS) and the central nervous system (CNS) by the functional mechanisms involved, especially in the extent and speed of repair. When an axon is damaged, the distal segment undergoes Wallerian degeneration, losing its myelin sheath. The proximal segment can either die by apoptosis or undergo the chromatolytic reaction, which is an attempt at repair. In the CNS, synaptic stripping occurs as glial foot processes invade the dead synapse.
Neural tissue engineering is a specific sub-field of tissue engineering. Neural tissue engineering is primarily a search for strategies to eliminate inflammation and fibrosis upon implantation of foreign substances. Often foreign substances in the form of grafts and scaffolds are implanted to promote nerve regeneration and to repair damage caused to nerves of both the central nervous system (CNS) and peripheral nervous system (PNS) by an injury.

Olfactory ensheathing cells (OECs), also known as olfactory ensheathing glia or olfactory ensheathing glial cells, are a type of macroglia found in the nervous system. They are also known as olfactory Schwann cells, because they ensheath the non-myelinated axons of olfactory neurons in a similar way to which Schwann cells ensheath non-myelinated peripheral neurons. They also share the property of assisting axonal regeneration.
Regeneration in humans is the regrowth of lost tissues or organs in response to injury. This is in contrast to wound healing, or partial regeneration, which involves closing up the injury site with some gradation of scar tissue. Some tissues such as skin, the vas deferens, and large organs including the liver can regrow quite readily, while others have been thought to have little or no capacity for regeneration following an injury.
Paweł Tabakow is a Polish neurosurgeon who is known for prepared and performing the operation that allowed Darek Fidyka to recover sensory and motor function after the complete severing of his spinal cord. Tabakow has claimed that an Indian ambassador and other people from round the world have contacted him about performing similar treatments.
Mary Elizabeth Bartlett Bunge was an American neuroscientist who researched a cure for paralysis at the University of Miami, where she was a professor of cell biology.
Nano neuro knitting is an emerging technology for repairing nervous system tissues via nano scaffolding techniques. Currently being explored in numerous research endeavors, nano neuro knitting has been shown to allow partial reinnervation in damaged areas of the nervous system through the interactions between potentially regenerative axons and peptide scaffolds. This interaction has been shown to lead to sufficient axon density renewal to the point that functionality is restored. While nano neuro knitting shows promise, the uncertainty of the effects in human subjects warrants further investigation before clinical trials initiate.









