Bone marrow is a semi-solid tissue found within the spongy portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production. It is composed of hematopoietic cells, marrow adipose tissue, and supportive stromal cells. In adult humans, bone marrow is primarily located in the ribs, vertebrae, sternum, and bones of the pelvis. Bone marrow comprises approximately 5% of total body mass in healthy adult humans, such that a man weighing 73 kg (161 lbs) will have around 3.7 kg (8 lbs) of bone marrow.

Adipose tissue is a loose connective tissue composed mostly of adipocytes. It also contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and a variety of immune cells such as adipose tissue macrophages. Its main role is to store energy in the form of lipids, although it also cushions and insulates the body.

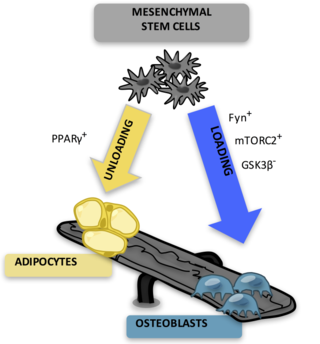
Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. In cell culture, adipocyte progenitors can also form osteoblasts, myocytes and other cell types.
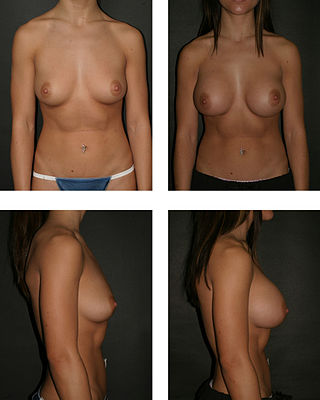
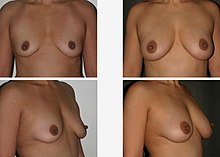
Breast augmentation and augmentation mammoplasty is a cosmetic surgery technique using breast-implants and fat-graft mammoplasty techniques to increase the size, change the shape, and alter the texture of the breasts. Augmentation mammoplasty is applied to correct congenital defects of the breasts and the chest wall. As an elective cosmetic surgery, primary augmentation changes the aesthetics – of size, shape, and texture – of healthy breasts.

Cell therapy is a therapy in which viable cells are injected, grafted or implanted into a patient in order to effectuate a medicinal effect, for example, by transplanting T-cells capable of fighting cancer cells via cell-mediated immunity in the course of immunotherapy, or grafting stem cells to regenerate diseased tissues.
Stromal cells, or mesenchymal stromal cells, are differentiating cells found in abundance within bone marrow but can also be seen all around the body. Stromal cells can become connective tissue cells of any organ, for example in the uterine mucosa (endometrium), prostate, bone marrow, lymph node and the ovary. They are cells that support the function of the parenchymal cells of that organ. The most common stromal cells include fibroblasts and pericytes. The term stromal comes from Latin stromat-, "bed covering", and Ancient Greek στρῶμα, strôma, "bed".

Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells, they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.
Stem-cell therapy uses stem cells to treat or prevent a disease or condition. As of 2016, the only established therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone marrow transplantation, but the cells can also be derived from umbilical cord blood. Research is underway to develop various sources for stem cells as well as to apply stem-cell treatments for neurodegenerative diseases and conditions such as diabetes and heart disease.
Articular cartilage, most notably that which is found in the knee joint, is generally characterized by very low friction, high wear resistance, and poor regenerative qualities. It is responsible for much of the compressive resistance and load bearing qualities of the knee joint and, without it, walking is painful to impossible. Osteoarthritis is a common condition of cartilage failure that can lead to limited range of motion, bone damage and invariably, pain. Due to a combination of acute stress and chronic fatigue, osteoarthritis directly manifests itself in a wearing away of the articular surface and, in extreme cases, bone can be exposed in the joint. Some additional examples of cartilage failure mechanisms include cellular matrix linkage rupture, chondrocyte protein synthesis inhibition, and chondrocyte apoptosis. There are several different repair options available for cartilage damage or failure.
Mesenchymal stem cells (MSCs) are multipotent cells found in multiple human adult tissues, including bone marrow, synovial tissues, and adipose tissues. Since they are derived from the mesoderm, they have been shown to differentiate into bone, cartilage, muscle, and adipose tissue. MSCs from embryonic sources have shown promise scientifically while creating significant controversy. As a result, many researchers have focused on adult stem cells, or stem cells isolated from adult humans that can be transplanted into damaged tissue.
Amniotic stem cells are the mixture of stem cells that can be obtained from the amniotic fluid as well as the amniotic membrane. They can develop into various tissue types including skin, cartilage, cardiac tissue, nerves, muscle, and bone. The cells also have potential medical applications, especially in organ regeneration.
MIRA is a multidisciplinary and complementary method for treating many chronic diseases. The MIRA Procedure is a result of combining efforts from different medical fields developed in the University of Chicago in 1992. It basically consists in medically grafting live rejuvenated tissue in the form of autologous adipose adult stem cells to a damaged organ in order to restore it and improve its function. This method is currently approved by the U.S. Food and Drug Administration (FDA).

Mesenchymal stem cells (MSCs) also known as mesenchymal stromal cells or medicinal signaling cells, are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes and adipocytes.
Adult mesenchymal stem cells are being used by researchers in the fields of regenerative medicine and tissue engineering to artificially reconstruct human tissue which has been previously damaged. Mesenchymal stem cells are able to differentiate, or mature from a less specialized cell to a more specialized cell type, to replace damaged tissues in various organs.
A Muse cell is an endogenous non-cancerous pluripotent stem cell. They reside in the connective tissue of nearly every organ including the umbilical cord, bone marrow and peripheral blood. They are collectable from commercially obtainable mesenchymal cells such as human fibroblasts, bone marrow-mesenchymal stem cells and adipose-derived stem cells. Muse cells are able to generate cells representative of all three germ layers from a single cell both spontaneously and under cytokine induction. Expression of pluripotency genes and triploblastic differentiation are self-renewable over generations. Muse cells do not undergo teratoma formation when transplanted into a host environment in vivo. This can be explained in part by their intrinsically low telomerase activity, eradicating the risk of tumorigenesis through unbridled cell proliferation. They were discovered in 2010 by Mari Dezawa and her research group. Clinical trials for acute myocardial infarction, stroke, epidermolysis bullosa, spinal cord injury, amyotrophic lateral sclerosis, acute respiratory distress syndrome (ARDS) related to novel coronavirus (SARS-CoV-2) infection, are conducted by Life Science Institute, Inc., a group company of Mitsubishi Chemical Holdings company. In february 2023, however, Mitsubishi Chemical Group decided to discontinue the development of a regenerative medicine product (CL2020) using Muse Cells. Physician-led clinical trial for neonatal hypoxic-ischemic encephalopathy was also started. The summary results of a randomized double-blind placebo-controlled clinical trial in patients with stroke was announced.
Facial Autologous Muscular Injection is also known as Fat Autograft Muscular Injection, as Autologous Fat Injection, as Micro-lipoinjection, as Fat Transfer and as Facial Autologous Mesenchymal Integration, abbreviated as FAMI. The technique is a non-incisional pan-facial rejuvenation procedure using the patient'own stem cells from fat deposits. FAMI is an Adult stem cell procedure used to address the loss of volume in the face due to aging or surgery repair in restoring facial muscles, bone surfaces and very deep fat pads. The procedure involves removing adult stem cells of fatty tissue from lower body, and refining it to be able to re-inject living adipose stem cells into specific areas of the face without incision. FAMI is an outpatient procedure and an alternative to artificial fillers, blepharoplasty or various face lifts. The procedure does not require general anesthesia and risks of an allergic reaction are minimal due to the use of the patient's own tissue used as the facial injection.
Bone marrow adipose tissue (BMAT), sometimes referred to as marrow adipose tissue (MAT), is a type of fat deposit in bone marrow. It increases in states of low bone density -osteoporosis, anorexia nervosa/caloric restriction, skeletal unweighting such as that which occurs in space travel, and anti-diabetes therapies. BMAT decreases in anaemia, leukaemia, and hypertensive heart failure; in response to hormones such as oestrogen, leptin, and growth hormone; with exercise-induced weight loss or bariatric surgery; in response to chronic cold exposure; and in response to pharmacological agents such as bisphosphonates, teriparatide, and metformin.

Shimon Slavin is an Israeli professor of medicine. Slavin pioneered the use of immunotherapy mediated by allogeneic donor lymphocytes and innovative methods for stem cell transplantation for the cure of hematological malignancies and solid tumors, and using hematopoietic stem cells for induction of transplantation tolerance to bone marrow and donor allografts.
Craniofacial regeneration refers to the biological process by which the skull and face regrow to heal an injury. This page covers birth defects and injuries related to the craniofacial region, the mechanisms behind the regeneration, the medical application of these processes, and the scientific research conducted on this specific regeneration. This regeneration is not to be confused with tooth regeneration. Craniofacial regrowth is broadly related to the mechanisms of general bone healing.
Fat transfer, also known as fat graft, lipomodelling, or fat injections, is a surgical process in which a person's own fat is transferred from one area of the body to another area. The major aim of this procedure is to improve or augment the area that has irregularities and grooves. Carried out under either general anesthesia or local anesthesia, the technique involves 3 main stages: fat harvesting, fat processing, fat injection.