
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.

Lysine is an α-amino acid that is a precursor to many proteins. It contains an α-amino group, an α-carboxylic acid group, and a side chain lysyl, classifying it as a basic, charged, aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.

Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the l-arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production.

Anabolism is the set of metabolic pathways that construct macromolecules like DNA or RNA from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking-down aspect. Anabolism is usually synonymous with biosynthesis.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.

The rove beetles are a family (Staphylinidae) of beetles, primarily distinguished by their short elytra that typically leave more than half of their abdominal segments exposed. With over 66,000 species in thousands of genera, the group is the largest family in the beetle order, and one of the largest families of organisms. It is an ancient group, with fossilized rove beetles known from the Triassic, 200 million years ago, and possibly even earlier if the genus Leehermania proves to be a member of this family. They are an ecologically and morphologically diverse group of beetles, and commonly encountered in terrestrial ecosystems.
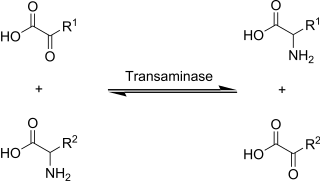
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids.This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids.
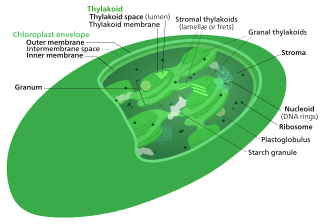
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation (redox) reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of CO2 to a sugar. There are three phases to the light-independent reactions, collectively called the Calvin cycle: carboxylation, reduction reactions, and ribulose 1,5-bisphosphate (RuBP) regeneration.

Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Amino acid biosynthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule.

Tyrosine aminotransferase is an enzyme present in the liver and catalyzes the conversion of tyrosine to 4-hydroxyphenylpyruvate.

Polylysine refers to several types of lysine homopolymers, which may differ from each other in terms of stereochemistry and link position (α/ε). Of these types, only ε-poly-L-lysine is produced naturally.

Stenus is a genus of semiaquatic rove beetles in the subfamily Steninae, and one of the largest genera in the kingdom Animalia, with some 2700 known species worldwide. They are predators of Collembola and other small arthropods. Adults have a protrusible labium with a sticky tip used in prey capture. To overcome the rapid escape of Collembola, the labium is protruded at high speed by hemolymph pressure, and immediately withdrawn, pulling the prey within the range of the mandibles. However, the labium tip does not easily stick to prey covered in scales or setae or that have a large body size. Stenus comma is more likely to catch such prey by lunging forward and grabbing them directly with its mandibles rather than using its labium. Stenus species are also known for "skimming" on the water surface using their pygidial gland secretions that act as a surfactant and rapidly propel the beetle fast forward, a phenomenon known as the Marangoni effect. Stenus comma has been seen to achieve a velocity of 0.75 m/s, and to cover a distance of up to 15 m if the secretion is continuous.

Paederus is a genus of small beetles of the family Staphylinidae. With 622 valid species assigned by 1987 to the subtribe Paederina, and with all but 148 within Paederus itself, the genus is large. Due to toxins in the hemolymph of some species within this genus, it has given its name to paederus dermatitis, a characteristic skin irritation that occurs if one of the insects is crushed against skin. That name, Paederus dermatitis, is a poor choice because, decades earlier, the affliction had been called dermatitis linearis, a name that works in all languages, not just English, because of its Latin origin; the name Paederus dermatitis is also inappropriate because it has shown to be caused by (a) only a few species of the genus Paederus, but (b) also a few species that belong to closely related genera within the subtribe Paederina. A scholarly paper in 2002 suggested that a Paederus species could have been responsible for some of the ten Plagues of Egypt described in the Bible's Book of Exodus.
Rearrangements, especially those that can participate in cascade reactions, such as the aza-Cope rearrangements, are of high practical as well as conceptual importance in organic chemistry, due to their ability to quickly build structural complexity out of simple starting materials. The aza-Cope rearrangements are examples of heteroatom versions of the Cope rearrangement, which is a [3,3]-sigmatropic rearrangement that shifts single and double bonds between two allylic components. In accordance with the Woodward-Hoffman rules, thermal aza-Cope rearrangements proceed suprafacially. Aza-Cope rearrangements are generally classified by the position of the nitrogen in the molecule :

Stenus melanarius is a species of rove beetle widely spread in Asia and Europe. It is a natural predator of the pest, Cnaphalocrocis medinalis.
Stenus solutus is a species of rove beetle widely spread in Asia and England.

















