Related Research Articles

Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.

α-Linolenic acid, also known as alpha-Linolenic acid (ALA), is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.

Tallow is a rendered form of beef or mutton suet, primarily made up of triglycerides.
Triadica sebifera is a tree native to eastern China. It is commonly called Chinese tallow, Chinese tallowtree, Florida aspen, chicken tree, gray popcorn tree, or candleberry tree.

Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega-9 fatty acid, abbreviated with a lipid number of 18:1 cis-9, and a main product of Δ9-desaturase. It has the formula CH3−(CH2)7−CH=CH−(CH2)7−COOH. The name derives from the Latin word oleum, which means oil. It is the most common fatty acid in nature. The salts and esters of oleic acid are called oleates. It is part of many oils and thus used in a lot of artificial food, as well as for soap.

Omega-6 fatty acids are a family of polyunsaturated fatty acids that have in common a final carbon-carbon double bond in the n-6 position, that is, the sixth bond, counting from the methyl end.

A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air, at room temperature. The oil hardens through a chemical reaction in which the components crosslink by the action of oxygen. Drying oils are a key component of oil paint and some varnishes. Some commonly used drying oils include linseed oil, tung oil, poppy seed oil, perilla oil, and walnut oil. Their use has declined over the past several decades, as they have been replaced by alkyd resins and other binders.
Linoleic acid (LA) is an organic compound with the formula HOOC(CH
2)
7CH=CHCH
2CH=CH(CH
2)
4CH
3. Both alkene groups are cis. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.

Saponification value or saponification number represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value are more suitable for soap making.

Sunflower oil is the non-volatile oil pressed from the seeds of the sunflower. Sunflower oil is commonly used in food as a frying oil, and in cosmetic formulations as an emollient.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linolenic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.

Perilla oil is an edible vegetable oil derived from perilla seeds. Having a distinct nutty aroma and taste, the oil pressed from the toasted perilla seeds is used as a flavor enhancer, condiment, and cooking oil in Korean cuisine. The oil pressed from untoasted perilla seeds is used for non-culinary purposes.
Candlenut oil or kukui nut oil is extracted from the nut of Aleurites moluccanus, the candlenut or kuku'i.
Cyclopropane fatty acids (CPA) are a subgroup of fatty acids that contain a cyclopropane group. Although they are usually rare, the seed oil from lychee contains nearly 40% CPAs in the form of triglycerides.

α-Parinaric acid is a conjugated polyunsaturated fatty acid. Discovered by Tsujimoto and Koyanagi in 1933, it contains 18 carbon atoms and 4 conjugated double bonds. The repeating single bond-double bond structure of α-parinaric acid distinguishes it structurally and chemically from the usual "methylene-interrupted" arrangement of polyunsaturated fatty acids that have double-bonds and single bonds separated by a methylene unit (−CH2−). Because of the fluorescent properties conferred by the alternating double bonds, α-parinaric acid is commonly used as a molecular probe in the study of biomembranes.
Vateria indica oil is extracted from the seeds of the Vateria indica plant, a species in the family Dipterocarpaceae. The Vateria indica plant is indigenous to the Western Ghats, Kerala and Tamil Nadu regions of India. It thrives in the evergreen forests, surviving up to 800 meters above sea level. Oil from the seeds of the plant is extracted through a chemical refining process which makes the plant edible.
Triadica cochinchinensis is a species of tree known as the mountain tallow tree.
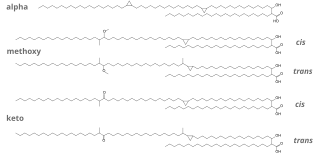
Stillingia tallow or Chinese vegetable tallow is a fatty substance extracted from the coat of the seeds of Triadica sebifera or Triadica cochinchinensis. It has traditionally been used for making candles. This product must be distinguished from stillingia oil, that is extracted from the seeds of those trees.
Edible oil refining is a set of processes or treatments necessary to turn vegetable raw oil into edible oil.
References
- ↑ Duke, James A. (1982). "{{{section}}}". Handbook of Energy Crops. Purdue Center for New Crops.
- 1 2 3 4 A. Crossley and T. P. Hilditch (1950): "The component acids of some authentic and commercial stillingia oils". Journal of the Science of Food and Agriculture, volume 1, issue 10, pages 292–300. doi : 10.1002/jsfa.2740011003
- 1 2 J. Devine (1950): "The composition of stillingia oil and the presence therein of 2:4-decadienoic acid". Journal of the Science of Food and Agriculture, volume 1, issue 3, pages 88–92. doi : 10.1002/jsfa.2740010307
- ↑ FAO (1994): "Vegetable and animal oils and fats". Chapter in Definition and Classification of Commodities. Accessed on 2006-11-19.
- 1 2 3 4 5 B. S. J. Jeffrey F. B. Padley (1991): "Chinese vegetable tallow - Characterization and contamination by stillingia oil". Journal of the American Oil Chemists' Society, doi : 10.1007/BF02662332
- ↑ Hans-Joachim Esser (2002): "A revision of Triadica Lour. (Euphorbiaceae)". Harvard Papers in Botany, volume 7, issue 1, pages 17-21 (5 pages)
- 1 2 3 4 5 Yun Liu, Hong-lingXin, and Yun-junYan (2009): "Physicochemical properties of stillingia oil: Feasibility for biodiesel production by enzyme transesterification". Industrial Crops and Products, volume 30, issue 3, pages 431-436. doi : 10.1016/j.indcrop.2009.08.004
- 1 2 V. C. Batterson, and W. M. Potts (1938-11-01). "Stillingia oil". Oil & Soap. 15 (11): 295–296. doi:10.1007/BF02642910. ISSN 1558-9331. S2CID 198138852.
- ↑ Ya-Chi Chen, A. Zlatkis, B. S. Middleditch, J. Cowles, and W. Scheld (1987): "Lipids of contemporary stillingia oil". Chromatographia, volume 23, pages 240–242. doi : 10.1007/BF02311771
- 1 2 3 A. Crossley and T. P. Hilditch (1953): "The component glycerides of stillingia oil". Journal of the Science of Food and Agriculture, volume 4, pages 38–44, doi : 10.1002/jsfa.2740040107
- ↑ K. Aitzetmüller, Yaonian Xin, Gisela Werner, and Margaretha Grönheim (1992): "High-performance liquid chromatographic investigations of stillingia oil". Journal of Chromatography A, volume 603, issues 1–2, pages 165-173. doi : 10.1016/0021-9673(92)85358-Z