Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.
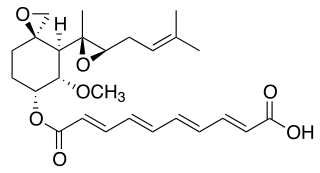
Fumagillin is a complex biomolecule and used as an antimicrobial agent. It was isolated in 1949 from the microbial organism Aspergillus fumigatus.

Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel (Taxol). This diterpenoid is an important drug in the treatment of cancer but, also expensive because the compound is harvested from a scarce resource, namely the Pacific yew. Not only is the synthetic reproduction of the compound itself of great commercial and scientific importance, but it also opens the way to paclitaxel derivatives not found in nature but with greater potential.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the metal catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.

Alstonia scholaris, commonly called blackboard tree, Scholar Tree, Milkwood or devil's tree in English, is an evergreen tropical tree in the Dogbane Family (Apocynaceae). It is native to southern China, tropical Asia (mainly the Indian subcontinent and Southeast Asia)and Australasia, where it is a common ornamental plant. It is a toxic plant, but is used traditionally for myriad diseases and complaints.
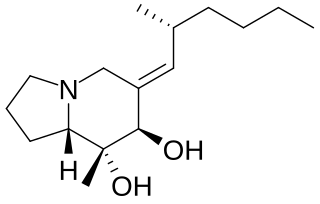
Allopumiliotoxin 267A is a toxin found in the skin of several poison frogs of the family Dendrobates. It is a member of the class of compounds known as allopumiliotoxins. The frogs produce the toxin by modifying the original version, pumiliotoxin 251D. It has been tested on mice and found to be five times more potent than the former version. It has been produced synthetically through a variety of different routes.

Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs methorphan, buprenorphine, hydromorphone, hydrocodone, isocodeine, naltrexone, nalbuphine, oxymorphone, oxycodone, and naloxone.

Gelsemine (C20H22N2O2) is an indole alkaloid isolated from flowering plants of the genus Gelsemium, a plant native to the subtropical and tropical Americas, and southeast Asia, and is a highly toxic compound that acts as a paralytic, exposure to which can result in death. It has generally potent activity as an agonist of the mammalian glycine receptor, the activation of which leads to an inhibitory postsynaptic potential in neurons following chloride ion influx, and systemically, to muscle relaxation of varying intensity and deleterious effect. Despite its danger and toxicity, recent pharmacological research has suggested that the biological activities of this compound may offer opportunities for developing treatments related to xenobiotic or diet-induced oxidative stress, and of anxiety and other conditions, with ongoing research including attempts to identify safer derivatives and analogs to make use of gelsemine's beneficial effects.

N-Sulfinyl imines are a class of imines bearing a sulfinyl group attached to nitrogen. These imines display useful stereoselectivity reactivity and due to the presence of the chiral electron withdrawing N-sulfinyl group. They allow 1,2-addition of organometallic reagents to imines. The N-sulfinyl group exerts powerful and predictable stereodirecting effects resulting in high levels of asymmetric induction. Racemization of the newly created carbon-nitrogen stereo center is prevented because anions are stabilized at nitrogen. The sulfinyl chiral auxiliary is readily removed by simple acid hydrolysis. The addition of organometallic reagents to N-sulfinyl imines is the most reliable and versatile method for the asymmetric synthesis of amine derivatives. These building blocks have been employed in the asymmetric synthesis of numerous biologically active compounds.

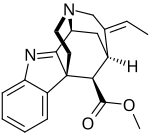
Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Kopsanone is an alkaloid isolated from Aspidosperma.

(-)-Versicolamide B and (+)-Versicolamide B are spiroindole alkaloids isolated from the fungus Aspergillus that belong to a class of naturally occurring 2,5-diketopiperazines. The versicolamides are structurally complex spiro-cyclized versions of prenylated cyclo(L-Trp-L-Pro) derivatives which possess a unique spiro-fusion to a pyrrolidine at the 3-position of the oxindole core together with the bicyclo[2.2.2]diazaoctane ring system. While (-)-versicolamide B was isolated from the marine fungus Aspergillus sp. the enantiomer (+)-versicolamide B was isolated from the terrestrial fungi Aspergillus versicolor NRRL. The total asymmetric syntheses of both enantiomers have been achieved and the implications of their biosynthesis have been investigated.
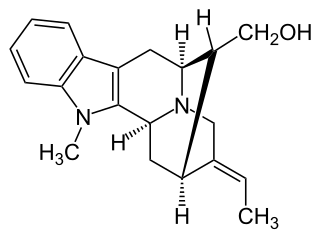
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.

The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols.
Vibralactones are a group of related terpenoid chemical compounds, the first of which were isolated from the Basidiomycete Boreostereum vibrans in 2006. Additional vibralactones have also been isolated from the fungus Stereum hirsutum.
Vinylcyclopropane [5+2] cycloaddition is a type of cycloaddition between a vinylcyclopropane (VCP) and an olefin or alkyne to form a seven-membered ring.
Selenogallates are chemical compounds which contain anionic units of selenium connected to gallium. They can be considered as gallates where selenium substitutes for oxygen. Similar compounds include the thiogallates and selenostannates. They are in the category of chalcogenotrielates or more broadly chalcogenometallates.
Sulfidostannates, or thiostannates are chemical compounds containing anions composed of tin linked with sulfur. They can be considered as stannates with sulfur substituting for oxygen. Related compounds include the thiosilicates, and thiogermanates, and by varying the chalcogen: selenostannates, and tellurostannates. Oxothiostannates have oxygen in addition to sulfur. Thiostannates can be classed as chalcogenidometalates, thiometallates, chalcogenidotetrelates, thiotetrelates, and chalcogenidostannates. Tin is almost always in the +4 oxidation state in thiostannates, although a couple of mixed sulfides in the +2 state are known,
Sulfidogermanates or thiogermanates are chemical compounds containing anions with sulfur atoms bound to germanium. They are in the class of chalcogenidotetrelates. Related compounds include thiosilicates, thiostannates, selenidogermanates, telluridogermanates and selenidostannates.