
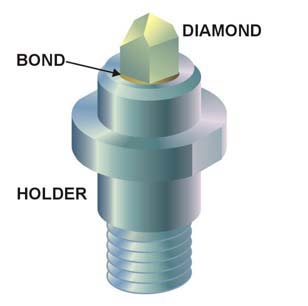
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.
The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

Silicon carbide (SiC), also known as carborundum, is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite.

Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond.

Titanium diboride (TiB2) is an extremely hard ceramic which has excellent heat conductivity, oxidation stability and wear resistance. TiB2 is also a reasonable electrical conductor, so it can be used as a cathode material in aluminium smelting and can be shaped by electrical discharge machining.
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.
A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some borides exhibit very useful physical properties. The term boride is also loosely applied to compounds such as B12As2 (N.B. Arsenic has an electronegativity higher than boron) that is often referred to as icosahedral boride.

A ferrite is an iron oxide-containing magnetic ceramic material. They are ferrimagnetic, meaning they are attracted by magnetic fields and can be magnetized to become permanent magnets. Unlike many ferromagnetic materials, most ferrites are not electrically conductive, making them useful in applications like magnetic cores for transformers to suppress eddy currents.
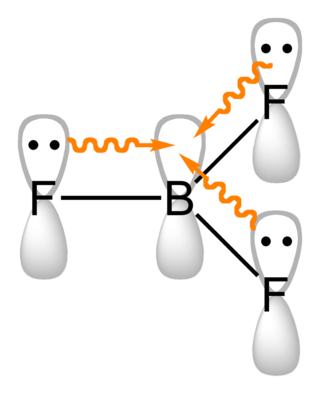
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides.
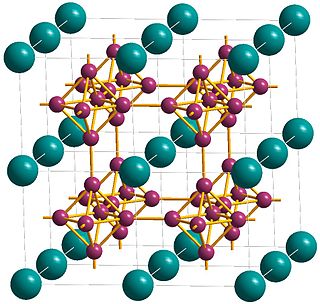
Calcium hexaboride (sometimes calcium boride) is a compound of calcium and boron with the chemical formula CaB6. It is an important material due to its high electrical conductivity, hardness, chemical stability, and melting point. It is a black, lustrous, chemically inert powder with a low density. It has the cubic structure typical for metal hexaborides, with octahedral units of 6 boron atoms combined with calcium atoms. CaB6 and lanthanum-doped CaB6 both show weak ferromagnetic properties, which is a remarkable fact because calcium and boron are neither magnetic, nor have inner 3d or 4f electronic shells, which are usually required for ferromagnetism.

Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra-high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ~6.09 g/cm3 (measured density may be higher due to hafnium impurities) and good high temperature strength makes it a candidate for high temperature aerospace applications such as hypersonic flight or rocket propulsion systems. It is an unusual ceramic, having relatively high thermal and electrical conductivities, properties it shares with isostructural titanium diboride and hafnium diboride.

Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV).
Metals, and specifically rare-earth elements, form numerous chemical complexes with boron. Their crystal structure and chemical bonding depend strongly on the metal element M and on its atomic ratio to boron. When B/M ratio exceeds 12, boron atoms form B12 icosahedra which are linked into a three-dimensional boron framework, and the metal atoms reside in the voids of this framework. Those icosahedra are basic structural units of most allotropes of boron and boron-rich rare-earth borides. In such borides, metal atoms donate electrons to the boron polyhedra, and thus these compounds are regarded as electron-deficient solids.
Silicon borides (also known as boron silicides) are lightweight ceramic compounds formed between silicon and boron. Several stoichiometric silicon boride compounds, SiBn, have been reported: silicon triboride, SiB3, silicon tetraboride, SiB4, silicon hexaboride, SiB6, as well as SiBn (n = 14, 15, 40, etc.). The n = 3 and n = 6 phases were reported as being co-produced together as a mixture for the first time by Henri Moissan and Alfred Stock in 1900 by briefly heating silicon and boron in a clay vessel. The tetraboride was first reported as being synthesized directly from the elements in 1960 by three independent groups: Carl Cline and Donald Sands; Ervin Colton; and Cyrill Brosset and Bengt Magnusson. It has been proposed that the triboride is a silicon-rich version of the tetraboride. Hence, the stoichiometry of either compound could be expressed as SiB4 - x where x = 0 or 1. All the silicon borides are black, crystalline materials of similar density: 2.52 and 2.47 g cm−3, respectively, for the n = 3(4) and 6 compounds. On the Mohs scale of mineral hardness, SiB4 - x and SiB6 are intermediate between diamond (10) and ruby (9). The silicon borides may be grown from boron-saturated silicon in either the solid or liquid state.

Chromium(III) boride, also known as chromium monoboride (CrB), is an inorganic compound with the chemical formula CrB. It is one of the six stable binary borides of chromium, which also include Cr2B, Cr5B3, Cr3B4, CrB2, and CrB4. Like many other transition metal borides, it is extremely hard (21-23 GPa), has high strength (690 MPa bending strength), conducts heat and electricity as well as many metallic alloys, and has a high melting point (~2100 °C). Unlike pure chromium, CrB is known to be a paramagnetic, with a magnetic susceptibility that is only weakly dependent on temperature. Due to these properties, among others, CrB has been considered as a candidate material for wear resistant coatings and high-temperature diffusion barriers.

Samarium hexaboride (SmB6) is an intermediate-valence compound where samarium is present both as Sm2+ and Sm3+ ions at the ratio 3:7. It is a Kondo insulator having a metallic surface state.
Ultra-high-temperature ceramics (UHTCs) are a type of refractory ceramics that can withstand extremely high temperatures without degrading, often above 2,000 °C. They also often have high thermal conductivities and are highly resistant to thermal shock, meaning they can withstand sudden and extreme changes in temperature without cracking or breaking. Chemically, they are usually borides, carbides, nitrides, and oxides of early transition metals.

Iron boride refers to various inorganic compounds with the formula FexBy. Two main iron borides are FeB and Fe2B. Some iron borides possess useful properties such as magnetism, electrical conductivity, corrosion resistance and extreme hardness. Some iron borides have found use as hardening coatings for iron. Iron borides have properties of ceramics such as high hardness, and properties of metal properties, such as thermal conductivity and electrical conductivity. Boride coatings on iron are superior mechanical, frictional, and anti-corrosive. Iron monoboride (FeB) is a grey powder that is insoluble in water. FeB is harder than Fe2B, but is more brittle and more easily fractured upon impact.
Samarium compounds are compounds formed by the lanthanide metal samarium (Sm). In these compounds, samarium generally exhibits the +3 oxidation state, such as SmCl3, Sm(NO3)3 and Sm(C2O4)3. Compounds with samarium in the +2 oxidation state are also known, for example SmI2.














