
Breast cancer is a cancer that develops from breast tissue. Signs of breast cancer may include a lump in the breast, a change in breast shape, dimpling of the skin, milk rejection, fluid coming from the nipple, a newly inverted nipple, or a red or scaly patch of skin. In those with distant spread of the disease, there may be bone pain, swollen lymph nodes, shortness of breath, or yellow skin.

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonist/antagonists (ERAAs), are a class of drugs that act on the estrogen receptor (ER). A characteristic that distinguishes these substances from pure ER agonists and antagonists is that their action is different in various tissues, thereby granting the possibility to selectively inhibit or stimulate estrogen-like action in various tissues.

Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome. Tamoxifen is typically taken daily by mouth for five years for breast cancer.
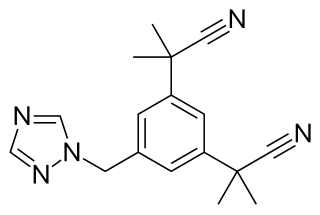
Aromatase inhibitors (AIs) are a class of drugs used in the treatment of breast cancer in postmenopausal women and in men, and gynecomastia in men. They may also be used off-label to reduce estrogen conversion when supplementing testosterone exogenously. They may also be used for chemoprevention in women at high risk for breast cancer.

Raloxifene, sold under the brand name Evista among others, is a medication used to prevent and treat osteoporosis in postmenopausal women and those on glucocorticoids. For osteoporosis it is less preferred than bisphosphonates. It is also used to reduce the risk of breast cancer in those at high risk. It is taken by mouth.

Letrozole, sold under the brand name Femara among others, is an aromatase inhibitor medication that is used in the treatment of breast cancer.
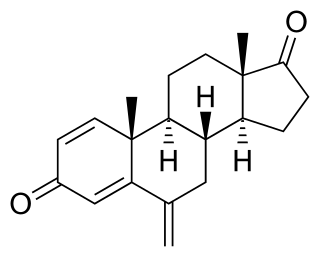
Exemestane, sold under the brand name Aromasin among others, is a medication used to treat breast cancer. It is a member of the class of antiestrogens known as aromatase inhibitors. Some breast cancers require estrogen to grow. Those cancers have estrogen receptors (ERs), and are called ER-positive. They may also be called estrogen-responsive, hormonally-responsive, or hormone-receptor-positive. Aromatase is an enzyme that synthesizes estrogen. Aromatase inhibitors block the synthesis of estrogen. This lowers the estrogen level, and slows the growth of cancers.

Toremifene, sold under the brand name Fareston among others, is a medication which is used in the treatment of advanced breast cancer in postmenopausal women. It is taken by mouth.

Virgil Craig Jordan,, is a scientist with American and British citizenship specializing in drugs for breast cancer treatment and prevention. Currently, he is Professor of Breast Medical Oncology, and Professor of Molecular and Cellular Oncology at the University of Texas MD Anderson Cancer Center, Houston, Texas. Previously, he was Scientific Director and Vice Chairman of Oncology at the Lombardi Comprehensive Cancer Center of Georgetown University. Jordan was the first to discover the breast cancer prevention properties of tamoxifen and the scientific principles for adjuvant therapy with antihormones. More recently his work has branched out into the prevention of multiple diseases in women with the discovery of the drug group, selective estrogen receptor modulator (SERMs). Currently, he plans to develop a new Hormone Replacement Therapy (HRT) for post-menopausal women that prevents breast cancer and does not increase the risk of breast cancer.

Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). It also appears to have had a statistically significant effect of reducing breast cancer in women according to a study published in The Journal of the National Cancer Institute.
Hormonal therapy in oncology is hormone therapy for cancer and is one of the major modalities of medical oncology, others being cytotoxic chemotherapy and targeted therapy (biotherapeutics). It involves the manipulation of the endocrine system through exogenous or external administration of specific hormones, particularly steroid hormones, or drugs which inhibit the production or activity of such hormones. Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing, or even undergo cell death. Surgical removal of endocrine organs, such as orchiectomy and oophorectomy can also be employed as a form of hormonal therapy.
Risk factors for breast cancer may be divided into preventable and non-preventable. Their study belongs in the field of epidemiology. Breast cancer, like other forms of cancer, can result from multiple environmental and hereditary risk factors. The term environmental, as used by cancer researchers, means any risk factor that is not genetically inherited.
AFPep is an orally-active, cyclic, 9-amino acid, peptide with a molecular weight of 969 daltons and is derived from the anti-oncogenic active site of alpha fetoprotein (AFP). Using the standard amino acid abbreviations, AFPep has the sequence cyclo(EKTOVNOGN), where O is hydroxyproline. This peptide has been shown in experimental animal models to be efficacious in the prevention and treatment of ER+ breast cancer.

Arzoxifene is a selective estrogen receptor modulator (SERM) of the benzothiophene group which was never marketed. It is a potent estrogen antagonist in mammary and uterine tissue while acting as an estrogen agonist to maintain bone density and lower serum cholesterol. Arzoxifene is a highly effective agent for prevention of mammary cancer induced in the rat by the carcinogen nitrosomethylurea and is significantly more potent than raloxifene in this regard. Arzoxifene is devoid of the uterotrophic effects of tamoxifen, suggesting that, in contrast to tamoxifen, it is unlikely that the clinical use of arzoxifene will increase the risk of developing endometrial carcinoma.
Hormone replacement therapy (HRT), also known as menopausal hormone therapy or postmenopausal hormone therapy, is a form of hormone therapy used to treat symptoms associated with female menopause. Effects of menopause can include symptoms such as hot flashes, accelerated skin aging, vaginal dryness, decreased muscle mass, and complications such as osteoporosis, sexual dysfunction, and vaginal atrophy. They are mostly caused by low levels of female sex hormones that occur during menopause.
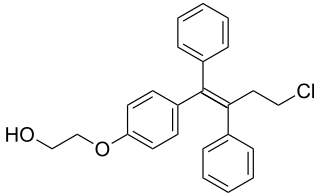
Ospemifene is an oral medication indicated for the treatment of dyspareunia – pain during sexual intercourse – encountered by some women, more often in those who are post-menopausal. Ospemifene is a selective estrogen receptor modulator (SERM) acting similarly to an estrogen on the vaginal epithelium, building vaginal wall thickness which in turn reduces the pain associated with dyspareunia. Dyspareunia is most commonly caused by "vulvar and vaginal atrophy."
Cancer prevention is the practice of taking active measures to decrease the incidence of cancer and mortality. The practice of prevention is dependent upon both individual efforts to improve lifestyle and seek preventive screening, and socioeconomic or public policy related to cancer prevention. Globalized cancer prevention is regarded as a critical objective due to its applicability to large populations, reducing long term effects of cancer by promoting proactive health practices and behaviors, and its perceived cost-effectiveness and viability for all socioeconomic classes.
Kathleen I. Pritchard, is the head of oncology at Sunnybrook Health Sciences Centre in Toronto, Canada, specializing in breast cancer therapies, and leading the clinical trials division of the centre. She has authored numerous studies on women's health, breast cancer, hormone replacement therapy, public health, and research methodology. According to Thomson Reuters, Pritchard was one of the most cited researchers in the world in 2014 and 2015.

Droloxifene, also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached phase II and phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials.

Worta J. McCaskill-Stevens was an American physician-scientist and medical oncologist specialized in cancer disparities research, management of comorbidities within clinical trials, and molecular research for cancer prevention interventions. She was chief of the community oncology and prevention trials research group at the National Cancer Institute.













