
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the ∞ symbol for a property of a solution denotes the property in the limit of infinite dilution." One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.

Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in its pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane.

Plasmolysis is the process in which cells lose water in a hypertonic solution. The reverse process, deplasmolysis or cytolysis, can occur if the cell is in a hypotonic solution resulting in a lower external osmotic pressure and a net flow of water into the cell. Through observation of plasmolysis and deplasmolysis, it is possible to determine the tonicity of the cell's environment as well as the rate solute molecules cross the cellular membrane.
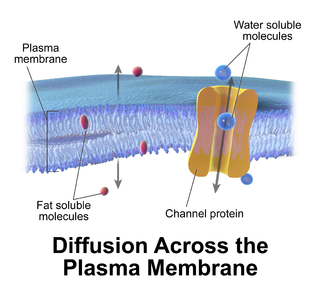
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to drive the movement of substances across cell membranes. Fundamentally, substances follow Fick's first law, and move from an area of high concentration to an area of low concentration because this movement increases the entropy of the overall system. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and/or osmosis.

In chemistry, colligative properties are those properties of solutions that depend on the ratio of the number of solute particles to the number of solvent particles in a solution, and not on the nature of the chemical species present. The number ratio can be related to the various units for concentration of a solution such as molarity, molality, normality (chemistry), etc. The assumption that solution properties are independent of nature of solute particles is exact only for ideal solutions, which are solutions that exhibit thermodynamic properties analogous to those of an ideal gas, and is approximate for dilute real solutions. In other words, colligative properties are a set of solution properties that can be reasonably approximated by the assumption that the solution is ideal.
The Starling principle holds that extracellular fluid movements between blood and tissues are determined by differences in hydrostatic pressure and colloid osmotic pressure between plasma inside microvessels and interstitial fluid outside them. The Starling equation, proposed many years after the death of Starling, describes that relationship in mathematical form and can be applied to many biological and non-biological semipermeable membranes. The classic Starling principle and the equation that describes it have in recent years been revised and extended.
Water potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure and matrix effects such as capillary action. The concept of water potential has proved useful in understanding and computing water movement within plants, animals, and soil. Water potential is typically expressed in potential energy per unit volume and very often is represented by the Greek letter ψ.

Soil moisture is the water content of the soil. It can be expressed in terms of volume or weight. Soil moisture measurement can be based on in situ probes or remote sensing methods.

In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution.

Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution. The osmolarity of a solution is usually expressed as Osm/L, in the same way that the molarity of a solution is expressed as "M".
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall.

A leaf sensor is a phytometric device that measures water loss or the water deficit stress (WDS) in plants by real-time monitoring the moisture level in plant leaves. The first leaf sensor was developed by LeafSens, an Israeli company granted a US patent for a mechanical leaf thickness sensing device in 2001. LeafSen has made strides incorporating their leaf sensory technology into citrus orchards in Israel. A solid state smart leaf sensor technology was developed by the University of Colorado at Boulder for NASA in 2007. It was designed to help monitor and control agricultural water demand. AgriHouse received a National Science Foundation (NSF) STTR grant in conjunction with the University of Colorado to further develop the solid state leaf sensor technology for precision irrigation control in 2007.
The pressure flow hypothesis, also known as the mass flow hypothesis, is the best-supported theory to explain the movement of sap through the phloem of plants. It was proposed in 1930 by Ernst Münch, a German plant physiologist. Organic molecules such as sugars, amino acids, certain hormones, and messenger RNAs are known to be transported in the phloem through the cells called sieve tube elements. According to the hypothesis, the high concentration of organic substances, particularly sugar, inside the phloem at a source such as a leaf creates a diffusion gradient that draws water into the cells from the adjacent xylem. This creates turgor pressure, also called hydrostatic pressure, in the phloem. The hypothesis states that this is why sap in plants flows from the sugar producers (sources) to sugar absorbers (sinks).

Osmosis is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential to a region of low water potential, in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to prevent net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity.
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes to keep the body fluids from becoming too diluted or concentrated. Osmotic pressure is a measure of the tendency of water to move into one solution from another by osmosis. The higher the osmotic pressure of a solution, the more water tends to move into it. Pressure must be exerted on the hypertonic side of a selectively permeable membrane to prevent diffusion of water by osmosis from the side containing pure water.
Osmotic dehydration is an operation used for the partial removal of water from plant tissues by immersion in a hypertonic (osmotic) solution.

In higher plants water and minerals are absorbed through root hairs which are in contact with soil water and from the root hairs zone a little the root tips.
Stomatal conductance, usually measured in mmol m−2 s−1 by a porometer, estimates the rate of gas exchange and transpiration through the leaf stomata as determined by the degree of stomatal aperture.
Leaf expansion is a process by which plants make efficient use of the space around them by causing their leaves to enlarge, or wither. This process enables a plant to maximize its own biomass, whether it be due to increased surface area; which enables more sunlight to be absorbed by chloroplasts, driving the rate of photosynthesis upward, or it enables more stomata to be created on the leaf surface, allowing the plant to increase its carbon dioxide intake.
Hydraulic signals in plants are detected as changes in the organism's water potential that are caused by environmental stress like drought or wounding. The cohesion and tension properties of water allow for these water potential changes to be transmitted throughout the plant.











