
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature.

Scabies is a contagious skin infestation by the mite Sarcoptes scabiei. The most common symptoms are severe itchiness and a pimple-like rash. Occasionally, tiny burrows may appear on the skin. In a first-ever infection, the infected person usually develops symptoms within two to six weeks. During a second infection, symptoms may begin within 24 hours. These symptoms can be present across most of the body or just certain areas such as the wrists, between fingers, or along the waistline. The head may be affected, but this is typically only in young children. The itch is often worse at night. Scratching may cause skin breakdown and an additional bacterial infection in the skin.

Hydrogen sulfide is a chemical compound with the formula H
2S. It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is stinkdamp. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. The British English spelling of this compound is hydrogen sulphide, a spelling no longer recommended by the Royal Society of Chemistry or the International Union of Pure and Applied Chemistry.
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.
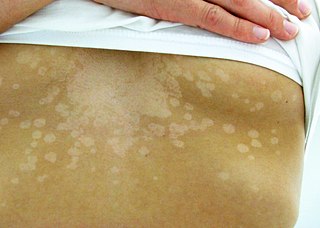
Tinea versicolor is a condition characterized by a skin eruption on the trunk and proximal extremities. The majority of tinea versicolor is caused by the fungus Malassezia globosa, although Malassezia furfur is responsible for a small number of cases. These yeasts are normally found on the human skin and become troublesome only under certain circumstances, such as a warm and humid environment, although the exact conditions that cause initiation of the disease process are poorly understood.

Demodicosis, also called Demodex folliculitis in humans and demodectic mange or red mange in animals, is caused by a sensitivity to and overpopulation of Demodex spp. as the host's immune system is unable to keep the mites under control.
Keratolytic therapy is a type of medical treatment to remove warts, calluses and other lesions in which the epidermis produces excess skin. In this therapy, acidic topical medicines, such as Whitfield's ointment or Jessner's solution, are applied to the lesion in order to thin the skin on and around it. This therapy causes the outer layer of the skin to loosen and shed.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
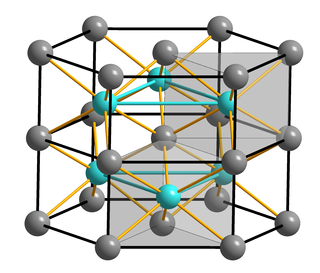
Iron(II) sulfide or ferrous sulfide is one of a family chemical compounds and minerals with the approximate formula FeS. Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids.

Ammonium bituminosulfonate or ammonium bituminosulphonate is a product of natural origin obtained in the first step by dry distillation of sulfur-rich oil shale. By sulfonation of the resulting oil, and subsequent neutralization with ammonia, Ichthammol results as a viscous, water-soluble substance with a characteristic bitumen-like odor. It is used in medicine as a treatment for different skin diseases, including eczema and psoriasis. Ointments containing 10% or 20% Ichthammol are most common. They are sometimes called "black ointments" or "drawing salves". Ichthammol's dermatological action was promoted by German physician Paul Gerson Unna.
Selenium disulfide, also known as selenium sulfide, is a chemical compound and medication used to treat seborrheic dermatitis, dandruff, and pityriasis versicolor. It is applied to the affected area as a lotion or shampoo. Symptoms frequently return if treatment is stopped.

Azelaic acid (AzA) is a organic compound with the formula HOOC(CH2)7COOH. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a precursor to diverse industrial products including polymers and plasticizers, as well as being a component of a number of hair and skin conditioners. AzA inhibits tyrosinase.

Ear mites are mites that live in the ears of animals and humans. The most commonly seen species in veterinary medicine is Otodectes cynotis. This species, despite its name, is also responsible for 90% of ear mite infections in felines.

Sulfur water is a condition where water is exposed to hydrogen sulfide gas, giving a distinct "rotten egg" smell. This condition has different purposes in culture varying to health and implications to plumbing.
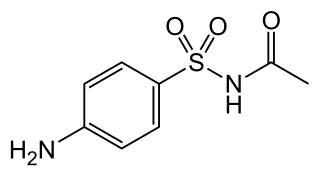
Sulfacetamide is a sulfonamide antibiotic.

Skin flora, also called skin microbiota, refers to microbiota that reside on the skin, typically human skin.
An ectoparasiticide is an antiparasitic drug used in the treatment of ectoparasitic infestations. These drugs are used to kill the parasites that live on the body surface. Permethrin, sulfur, lindane, dicophane, benzyl benzoate, ivermectin and crotamiton are well known ectoparasiticides.

A pimple is a kind of comedo that results from excess sebum and dead skin cells getting trapped in the pores of the skin. In its aggravated state, it may evolve into a pustule or papules. Pimples can be treated by acne medications, antibiotics, and anti-inflammatories prescribed by a physician, or various over the counter remedies purchased at a pharmacy.
Anti-seborrheics are drugs effective in seborrheic dermatitis. Selenium sulfide, zinc pyrithione, corticosteroids, imidazole antifungals, and salicylic acid are common anti-seborrheics.

The Isinuka Mud Caves and Sulphur Pools are located next to the Isinuka village which is located in OR Tambo District Municipality, Eastern Cape, South Africa. The name "Isinuka", means "place of smell", which refers to the odour of the sulfur which diffuses from the springs. This name was given by the Mpondo people of the Eastern Cape who regard this site as a sacred area. The springs are also visited by thousands of tourists who seek relief from various ailments yearly.















