
Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.
Locoweed is a common name in North America for any plant that produces swainsonine, an alkaloid harmful to livestock. Worldwide, swainsonine is produced by a small number of species, most of them in three genera of the flowering plant family Fabaceae: Oxytropis and Astragalus in North America, and Swainsona in Australia. The term locoweed usually refers only to the North American species of Oxytropis and Astragalus, but this article includes the other species as well. Some references may incorrectly list Datura as locoweed.
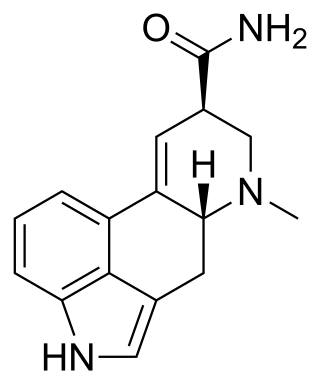
Ergine, also known as d-lysergic acid amide (LSA) and d-lysergamide, is an ergoline alkaloid that occurs in various species of vines of the Convolvulaceae and some species of fungi. The psychedelic properties in the seeds of ololiuhqui, Hawaiian baby woodrose and morning glories have been linked to ergine and/or isoergine, its epimer, as it is an alkaloid present in the seeds.

Lysergic acid, also known as D-lysergic acid and (+)-lysergic acid, is a precursor for a wide range of ergoline alkaloids that are produced by the ergot fungus and found in the seeds of Turbina corymbosa (ololiuhqui), Argyreia nervosa, and Ipomoea tricolor.

Astragalus is a large genus of over 3,000 species of herbs and small shrubs, belonging to the legume family Fabaceae and the subfamily Faboideae. It is the largest genus of plants in terms of described species. The genus is native to temperate regions of the Northern Hemisphere. Common names include milkvetch, locoweed and goat's-thorn. Some pale-flowered vetches are similar in appearance, but they are more vine-like than Astragalus.

Alpha-mannosidosis is a lysosomal storage disorder, first described by Swedish physician Okerman in 1967. In humans it is known to be caused by an autosomal recessive genetic mutation in the gene MAN2B1, located on chromosome 19, affecting the production of the enzyme alpha-D-mannosidase, resulting in its deficiency. Consequently, if both parents are carriers, there will be a 25% chance with each pregnancy that the defective gene from both parents will be inherited, and the child will develop the disease. There is a two in three chance that unaffected siblings will be carriers. In livestock alpha-mannosidosis is caused by chronic poisoning with swainsonine from locoweed.
Lycorine is a toxic crystalline alkaloid found in various Amaryllidaceae species, such as the cultivated bush lily, surprise lilies (Lycoris), and daffodils (Narcissus). It may be highly poisonous, or even lethal, when ingested in certain quantities. Regardless, it is sometimes used medicinally, a reason why some groups may harvest the very popular Clivia miniata.
Sparteine is a class 1a antiarrhythmic agent and sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in Lupinus mutabilis, and is thought to chelate the bivalent metals calcium and magnesium. It is not FDA approved for human use as an antiarrhythmic agent, and it is not included in the Vaughan Williams classification of antiarrhythmic drugs.

Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase is an enzyme that in humans is encoded by the MAN1B1 gene.

Alpha-mannosidase 2 is an enzyme that in humans is encoded by the MAN2A1 gene.

Mannosyl-oligosaccharide 1,2-alpha-mannosidase IA is an enzyme that in humans is encoded by the MAN1A1 gene.
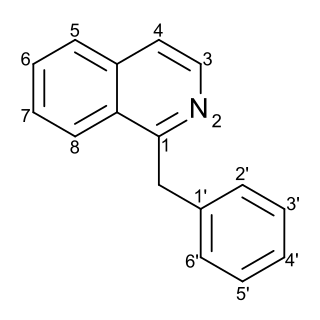
Substitution of the heterocycle isoquinoline at the C1 position by a benzyl group provides 1‑benzylisoquinoline, the most widely examined of the numerous benzylisoquinoline structural isomers. The 1-benzylisoquinoline moiety can be identified within numerous compounds of pharmaceutical interest, such as moxaverine; but most notably it is found within the structures of a wide variety of plant natural products, collectively referred to as benzylisoquinoline alkaloids. This class is exemplified in part by the following compounds: papaverine, noscapine, codeine, morphine, apomorphine, berberine, tubocurarine.

Tetrandrine, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. It is isolated from the plant Stephania tetrandra, and other Chinese and Japanese herbs.
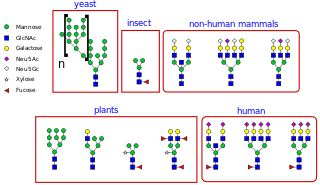
N-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan.

Ipomoea carnea, the pink morning glory, is a species of morning glory that grows as a bush. This flowering plant has heart-shaped leaves that are a rich green and 6–9 inches (15–23 cm) long. It can be easily grown from seeds. These seeds are toxic and it can be hazardous to cattle; the toxicity is related to the swainsonine produced by its endophytes, and to bioaccumulation of selenium in the leaves but mostly in the seeds.Ingestion of seeds or leaves causes abnormal endocrine functions and gastrointestinal functions, immune system alternation, abnormality in embryogenesis.

Castanospermine is an indolizidine alkaloid first isolated from the seeds of Castanospermum australe. It is a potent inhibitor of some glucosidase enzymes and has antiviral activity in vitro and in mouse models.

Methaneseleninic acid is an organoselenium compound, a seleninic acid with the chemical formula CH3SeO2H. Its structure is CH3−Se(=O)−OH.
Mannosyl-oligosaccharide 1,3-1,6-α-mannosidase, also known as Golgi α-mannosidase II, is an enzyme with systematic name (1→3)-(1→6)-mannosyl-oligosaccharide α-D-mannohydrolase. It catalyses the hydrolysis of the terminal (1→3)- and (1→6)-linked α-D-mannose residues in the mannosyl-oligosaccharide Man5(GlcNAc)3.

Kifunensine is an alkaloid originally isolated from Kitasatosporia kifunense, an actinobacterium. It is a neutral, stable compound.

(S)-Magnoflorine is a quaternary benzylisoquinoline alkaloid (BIA) of the aporphine structural subgroup which has been isolated from various species of the family Menispermaceae, such as Pachygone ovata,Sinomenium acutum, and Cissampelos pareira.




















