Cytochrome c oxidase copper chaperone is a protein that in humans is encoded by the COX17 gene.

ATP-dependent RNA helicase DDX24 is an enzyme that in humans is encoded by the DDX24 gene.

Elongation factor Tu, mitochondrial is a protein that in humans is encoded by the TUFM gene. It is an EF-Tu homolog.

Cadherin-6 is a protein that in humans is encoded by the CDH6 gene.

Isocitrate dehydrogenase [NAD] subunit gamma, mitochondrial is an enzyme that in humans is encoded by the IDH3G gene.

Transmembrane protein 50A is a protein that in humans is encoded by the TMEM50A gene.

Surfeit locus protein 6 is a protein that in humans is encoded by the SURF6 gene.

DGLUCY is a protein that in humans is encoded by the DGLUCY gene.

39S ribosomal protein L15, mitochondrial is a protein that in humans is encoded by the MRPL15 gene.

39S ribosomal protein L12, mitochondrial is a protein that in humans is encoded by the MRPL12 gene.
Transmembrane and ubiquitin-like domain-containing protein 2 is a protein that in humans is encoded by the TMUB2 gene.

Neuroblastoma breakpoint family, member 3, also known as NBPF3, is a human gene of the neuroblastoma breakpoint family, which resides on chromosome 1 of the human genome. NBPF3 is located at 1p36.12, immediately upstream of genes ALPL and RAP1GAP.

TRAPPC14 also known as MAP11 is a protein that in human is encoded by the gene TRAPPC14. It was previously referred to by the generic name C7orf43. C7orf43 has no other human alias, but in mice can be found as BC037034.

Transmembrane protein 222 is a protein that in humans is encoded by the TMEM222 gene. One notable feature of the protein encoded by this gene is the presence of three predicted transmembrane domains. The TMEM222 protein is predicted to most likely localize to the secretory vesicles.

Uncharacterized protein C1orf21, also known as Proliferation-Inducing Protein 13, is a protein that in humans is encoded by the C1orf21 gene. C1orf21 is an intracellular protein that flows between the nucleus and the cytoplasm in the cell. It has been linked with cell growth and reproduction and there has been strong links with various types of cancers. There are no paralogs for this gene, however, many conserved orthologs have been found in all invertebrates. C1orf21 has low to moderate level of expression in most tissues in humans, however, it has the most expression in the skin, lung and prostate.

Dual specificity protein phosphatase 12 is an enzyme that in humans is encoded by the DUSP12 gene.

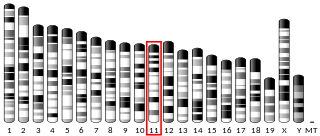
Chromosome 11 open reading frame one, also known as C11orf1, is a protein-coding gene. It has been found by yeast two hybrid screen to bind to SETDB1 a histone protein methyltransferase enzyme. SETDB1 has been implicated in Huntington's disease, a neurodegenerative disorder.
Transmembrane protein 126B is a protein that in humans is encoded by the TMEM126B gene. TMEM126B is a mitochondrial transmembrane protein which is a component of the mitochondrial complex I assembly complex. The TMEM126B gene is conserved in mammals. The encoded protein serves as an assembly factor that is required for formation of the membrane arm of the complex. It interacts with NADH dehydrogenase [ubiquinone] 1 alpha subcomplex assembly factor 13. Naturally occurring mutations in this gene are associated with isolated complex I deficiency. A pseudogene of this gene has been defined on chromosome 9.

MORN1 containing repeat 1, also known as Morn1, is a protein that in humans is encoded by the MORN1 gene.

Coiled-coil domain containing 60 is a protein that in humans is encoded by the CCDC60 gene that is most highly expressed in the trachea, salivary glands, bladder, cervix, and epididymis.
















