
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.

p53, also known as Tumor protein P53, cellular tumor antigen p53, or transformation-related protein 53 (TRP53) is a regulatory protein that is often mutated in human cancers. The p53 proteins are crucial in vertebrates, where they prevent cancer formation. As such, p53 has been described as "the guardian of the genome" because of its role in conserving stability by preventing genome mutation. Hence TP53 is classified as a tumor suppressor gene.

Tyrosine-protein kinase ABL1 also known as ABL1 is a protein that, in humans, is encoded by the ABL1 gene located on chromosome 9. c-Abl is sometimes used to refer to the version of the gene found within the mammalian genome, while v-Abl refers to the viral gene, which was initially isolated from the Abelson murine leukemia virus.

Protein kinase RNA-activated also known as protein kinase R (PKR), interferon-induced, double-stranded RNA-activated protein kinase, or eukaryotic translation initiation factor 2-alpha kinase 2 (EIF2AK2) is an enzyme that in humans is encoded by the EIF2AK2 gene on chromosome 2. PKR is a serine/tyrosine kinase that is 551 amino acids long.
Myc is a family of regulator genes and proto-oncogenes that code for transcription factors. The Myc family consists of three related human genes: c-myc (MYC), l-myc (MYCL), and n-myc (MYCN). c-myc was the first gene to be discovered in this family, due to homology with the viral gene v-myc.

Transcription factor Jun is a protein that in humans is encoded by the JUN gene. c-Jun, in combination with protein c-Fos, forms the AP-1 early response transcription factor. It was first identified as the Fos-binding protein p39 and only later rediscovered as the product of the JUN gene. c-jun was the first oncogenic transcription factor discovered. The proto-oncogene c-Jun is the cellular homolog of the viral oncoprotein v-jun. The viral homolog v-jun was discovered in avian sarcoma virus 17 and was named for ju-nana, the Japanese word for 17. The human JUN encodes a protein that is highly similar to the viral protein, which interacts directly with specific target DNA sequences to regulate gene expression. This gene is intronless and is mapped to 1p32-p31, a chromosomal region involved in both translocations and deletions in human malignancies.

Activator protein 1 (AP-1) is a transcription factor that regulates gene expression in response to a variety of stimuli, including cytokines, growth factors, stress, and bacterial and viral infections. AP-1 controls a number of cellular processes including differentiation, proliferation, and apoptosis. The structure of AP-1 is a heterodimer composed of proteins belonging to the c-Fos, c-Jun, ATF and JDP families.

Signal transducer and activator of transcription 5 (STAT5) refers to two highly related proteins, STAT5A and STAT5B, which are part of the seven-membered STAT family of proteins. Though STAT5A and STAT5B are encoded by separate genes, the proteins are 90% identical at the amino acid level. STAT5 proteins are involved in cytosolic signalling and in mediating the expression of specific genes. Aberrant STAT5 activity has been shown to be closely connected to a wide range of human cancers, and silencing this aberrant activity is an area of active research in medicinal chemistry.

Protein C-ets-1 is a protein that in humans is encoded by the ETS1 gene. The protein encoded by this gene belongs to the ETS family of transcription factors.

Colony stimulating factor 1 receptor (CSF1R), also known as macrophage colony-stimulating factor receptor (M-CSFR), and CD115, is a cell-surface protein encoded by the human CSF1R gene. CSF1R is a receptor that can be activated by two ligands: colony stimulating factor 1 (CSF-1) and interleukin-34 (IL-34). CSF1R is highly expressed in myeloid cells, and CSF1R signaling is necessary for the survival, proliferation, and differentiation of many myeloid cell types in vivo and in vitro. CSF1R signaling is involved in many diseases and is targeted in therapies for cancer, neurodegeneration, and inflammatory bone diseases.

MAX is a gene that in humans encodes the MAX transcription factor.

ERG is an oncogene. ERG is a member of the ETS family of transcription factors. The ERG gene encodes for a protein, also called ERG, that functions as a transcriptional regulator. Genes in the ETS family regulate embryonic development, cell proliferation, differentiation, angiogenesis, inflammation, and apoptosis.

Cell division protein kinase 8 is an enzyme that in humans is encoded by the CDK8 gene.
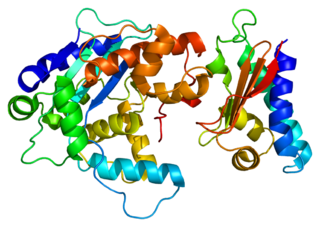
Protein Tob1 is a protein that in humans is encoded by the TOB1 gene.

Lysine-specific demethylase 5A is an enzyme that in humans is encoded by the KDM5A gene.

ETS domain-containing protein Elk-4 is a protein that in humans is encoded by the ELK4 gene.

THO complex subunit 1 is a protein that in humans is encoded by the THOC1 gene.
Post-transcriptional regulation is the control of gene expression at the RNA level. It occurs once the RNA polymerase has been attached to the gene's promoter and is synthesizing the nucleotide sequence. Therefore, as the name indicates, it occurs between the transcription phase and the translation phase of gene expression. These controls are critical for the regulation of many genes across human tissues. It also plays a big role in cell physiology, being implicated in pathologies such as cancer and neurodegenerative diseases.
Gene gating is a phenomenon by which transcriptionally active genes are brought next to nuclear pore complexes (NPCs) so that nascent transcripts can quickly form mature mRNA associated with export factors. Gene gating was first hypothesised by Günter Blobel in 1985. It has been shown to occur in Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster as well as mammalian model systems.
The TREX (TRanscription-EXport) complex is a conserved eukaryotic multi-protein complex that couples mRNA transcription and nuclear export. The TREX complex travels across transcribed genes with RNA polymerase II. TREX binds mRNA and recruits transport proteins NXF1 and NXT1, which shuttle the mRNA out of the nucleus. The TREX complex plays an important role in genome stability and neurodegenerative diseases.





















